Hi everyone,
I hope this message finds you all in good health and high spirits. This newsletter aims to be an educational resource, and your input is highly valued. If you have any specific aspects of the biotech industry you'd like us to explore, don't hesitate to send me a direct message, and I'll make sure to incorporate them into future editions.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
I would be grateful if you could help us spread the word about this newsletter among your biotech peers and colleagues. Our aim is to reach out to as many individuals as possible, fostering the growth of a well-informed community. This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
Your support for The Weekly Pill enables us to utilize costly software, providing you with the finest data. We are also introducing a paid exclusive section where we offer high-quality additional information. Join us as a paid subscriber today!
Quick question for you!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming catalysts next week
Most shorted biotech stocks
Earnings
Market comments
Notes
M&A comments
Q2 Biotech-focused funds moves
Conclusion
How the market performed this week
ETFs
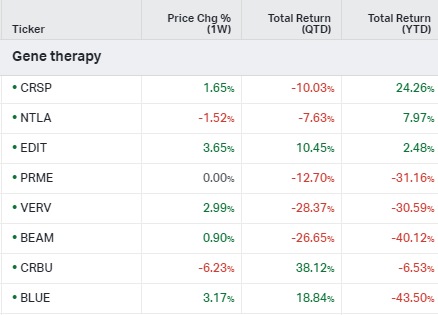
Gene Therapy
RNA Therapy
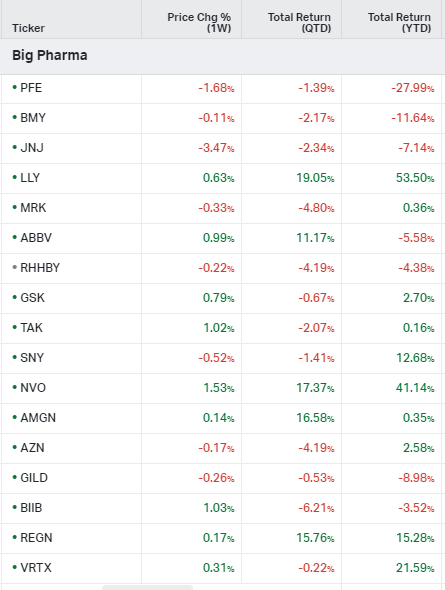
Big Pharma
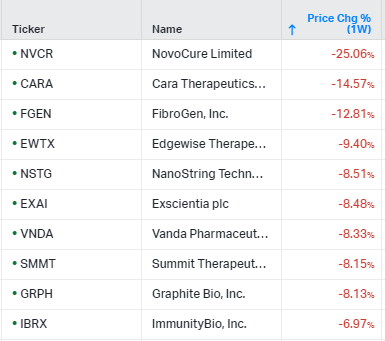
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Acer Therapeutics Inc. (ACER)
Event Phase: Approved
Drug: Olpruva
Indication: Urea Cycle Disorders and Derangements (UCD)
Lead Indication: N
Molecule: Small Molecule
Target: Glutamine|Tricarboxylic Acid (TCA) Cycle/Citric Acid Cycle (CAC)
LOA: 100%
Partner Companies: Relief Therapeutics Holding AG (RLF)
Source Link: Zevra Therapeutics to Acquire Acer Therapeutics: Expanding its Rare Disease Portfolio and Adding Commercial Product
Acer Therapeutics Inc. (ACER)
Event Phase: III
Drug: Edsivo
Indication: Metabolic - General
Lead Indication: Y
Molecule: Small Molecule
Target: Beta Adrenergic Receptors
LOA: 43%
Partner Companies: N/A
Source Link: Zevra Therapeutics to Acquire Acer Therapeutics: Expanding its Rare Disease Portfolio and Adding Commercial Product
Acer Therapeutics Inc. (ACER)
Event Phase: II
Drug: ACER-801
Indication: Neurology - Other
Lead Indication: Y
Molecule: Small Molecule
Target: Neurokinin Receptor
LOA: 12%
Partner Companies: Sanofi (SNY)
Source Link: Zevra Therapeutics to Acquire Acer Therapeutics: Expanding its Rare Disease Portfolio and Adding Commercial Product
Acer Therapeutics Inc. (ACER)
Event Phase: Preclinical
Drug: ACER-801
Indication: Post-Traumatic Stress Disorder (PTSD)
Lead Indication: N
Molecule: Small Molecule
Target: Neurokinin Receptor
Partner Companies: Sanofi (SNY)
Source Link: Zevra Therapeutics to Acquire Acer Therapeutics: Expanding its Rare Disease Portfolio and Adding Commercial Product
Acer Therapeutics Inc. (ACER)
Event Phase: II
Drug: ACER-801
Indication: Prostate Cancer
Lead Indication: N
Molecule: Small Molecule
Target: Neurokinin Receptor
LOA: 11%
Partner Companies: Sanofi (SNY)
Source Link: Zevra Therapeutics to Acquire Acer Therapeutics: Expanding its Rare Disease Portfolio and Adding Commercial Product
Acer Therapeutics Inc. (ACER)
Event Phase: I
Drug: Olpruva
Indication: Maple Syrup Urine Disease
Lead Indication: N
Molecule: Small Molecule
Target: Glutamine|Tricarboxylic Acid (TCA) Cycle/Citric Acid Cycle (CAC)
LOA: 15%
Partner Companies: Relief Therapeutics Holding AG (RLF)
Immune Therapeutics, Inc. (IMUN)
Event Phase: Preclinical
Drug: JKB-122
Indication: HIV / AIDS
Lead Indication: N
Molecule: Small Molecule
Target: Toll-like receptor 4 (TLR4)
Partner Companies: TaiwanJ Pharmaceuticals Co., Ltd. (6549)
Source Link: Biostax Corp Signs Collaboration Agreement with Immgenuity Inc to Pursue Remission in HIV
Immune Therapeutics, Inc. (IMUN)
Event Phase: Development Outside U.S.
Drug: Lodonal
Indication: HIV / AIDS
Lead Indication: N
Molecule: Small Molecule
Target: Opioid receptors
Partner Companies: Omaera Pharmaceuticals Ltd.|Statera BioPharma, Inc. (STAB)
Source Link: Biostax Corp Signs Collaboration Agreement with Immgenuity Inc to Pursue Remission in HIV
BioVaxys Technology Corp. (BVAXF)
Event Phase: Preclinical
Drug: BVX-1021
Indication: COVID-19 Prevention
Lead Indication: Y
Molecule: Cellular
Target: Immune System|SARS-CoV-2
Partner Companies: N/A
Source Link: Biovaxys and The Ohio State University Extend Research Collaboration
ImmunoGen, Inc. (IMGN)
Event Phase: Approved
Drug: Elahere
Indication: Ovarian Cancer
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Antibody-drug Conjugate (ADC)|Folate Receptor (FOLR1)|Microtubules (Tubulin)
LOA: 100%
Partner Companies: Huadong Medicine Co., Ltd. (000963)|Takeda Pharmaceutical Co. Ltd. (TAK)
Source Link: ImmunoGen Announces Collaboration with Huadong Medicine to Develop Elahere in Greater China
Small Pharma Ltd. (DMT)
Event Phase: Development Outside U.S.
Drug: SPL026
Indication: Major Depressive Disorder (MDD)
Lead Indication: Y
Molecule: Small Molecule
Target: Unknown
Partner Companies: N/A
Source Link: Small Pharma Announces Collaboration with Lucid Psycheceuticals to Develop SPL026 for Major Depressive Disorder
Small Pharma Ltd. (DMT)
Event Phase: Development Outside U.S.
Drug: SPL028
Indication: Major Depressive Disorder (MDD)
Lead Indication: N
Molecule: Small Molecule
Target: Unknown
Partner Companies: N/A
Source Link: Small Pharma Announces Collaboration with Lucid Psycheceuticals to Develop SPL028 for Major Depressive Disorder
Clinical trials (LOA=likelihood of approval)
Gain Therapeutics, Inc. (GANX)
Event Phase: Preclinical
Drug: GT-02287
Indication: Parkinson's Disease (PD)
Lead Indication: N
Molecule: Protein
Target: GBA2 (extralysosomal glucocerebrosidase)
Valneva SE (VLA)
Event Phase: NDA/BLA
Drug: VLA1553
Indication: Chikungunya Virus
Lead Indication: Y
Molecule: Vaccine
Target: Immune System
LOA: 97%
Partner Companies: Instituto Butantan
Source Link: Valneva Reports Positive Initial Phase 3 Safety Data in Adolescents for its Single Shot Chikungunya Vaccine Candidate
Bayer AG (BAYN)
Event Phase: I
Drug: BRT-DA01
Indication: Parkinson's Disease (PD)
Lead Indication: Y
Molecule: Cellular
Target: Dopamine|Stem Cells/Other Cell Therapies
LOA: 6%
Source Link: BlueRock's Phase I Study with Bemdaneprocel in Patients with Parkinson's Disease Meets Primary Endpoint
Novartis AG (NVS)
Event Phase: Approved
Drug: Leqvio
Indication: Dyslipidemia / Hypercholesterolemia
Lead Indication: Y
Molecule: siRNA/RNAi
Target: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9)
Bristol Myers Squibb Company (BMY)
Event Phase: Approved
Drug: Camzyos
Indication: Cardiomyopathy - Hypertrophic
Lead Indication: Y
Molecule: Small Molecule
Target: Myosin
Theravance Biopharma Inc. (TBPH)
Event Phase: III
Drug: TD-9855
Indication: Orthostatic Hypotension
Lead Indication: N
Molecule: Small Molecule
Target: Norepinephrine (Noradrenaline) Reuptake/Transporter|Serotonin Reuptake
LOA: 28%
Partner Companies: Royalty Pharma plc (RPRX)
BioVie, Inc. (BIVI)
Event Phase: II
Drug: NE3107
Indication: Parkinson's Disease (PD)
Lead Indication: N
Molecule: Small Molecule
Target: Tumor Necrosis Factor-alpha (TNF-alpha)
LOA: 12%
Source Link: BioVie Presents Data for NE3107 at 2023 International Congress of Parkinson's Disease and Movement Disorders
Neurocrine Biosciences, Inc. (NBIX)
Event Phase: Approved
Drug: Ingrezza
Indication: Huntington's Disease
Lead Indication: N
Molecule: Small Molecule
Target: Vesicular monoamine transporters (VMATs)
Roche Holding AG (RHHBY)
Event Phase: Approved
Drug: Alecensa
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: Y
Molecule: Small Molecule
Target: Anaplastic lymphoma kinase (ALK)
LOA: 100%
Partner Companies: Chugai Pharmaceutical Co., Ltd. (4519)
Source Link: Roche's Alecensa delivers unprecedented Phase III results for people with ALK-positive early-stage lung cancer
GSK plc (GSK)
Event Phase: Approved
Drug: Nucala Liquid Formulation
Indication: Nasal Polyposis
Lead Indication: N
Molecule: Monoclonal Antibody
Target: IL-5 (Interleukin-5) and IL-5 Receptor (IL-5R)
LOA: 100%
Partner Companies: PDL BioPharma, Inc. (PDLI)
Source Link: GSK's regulatory submission accepted for review by Japanese regulator for use of Nucala (mepolizumab)
GSK plc (GSK)
Event Phase: Approved
Drug: Nucala Liquid Formulation
Indication: Chronic Rhinosinusitis
Lead Indication: N
Molecule: Monoclonal Antibody
Target: IL-5 (Interleukin-5) and IL-5 Receptor (IL-5R)
LOA: 100%
Partner Companies: PDL BioPharma, Inc. (PDLI)
Source Link: GSK's regulatory submission accepted for review by Japanese regulator for use of Nucala (mepolizumab)
Bavarian Nordic A/S (BAVA)
Event Phase: III
Drug: ABNCoV2
Indication: COVID-19 Prevention
Lead Indication: Y
Molecule: Vaccine
Target: Immune System|SARS-CoV-2
LOA: 63%
Partner Companies: AdaptVac
Source Link: Bavarian Nordic Provides Update on COVID-19 Booster Vaccine Program
Intellia Therapeutics, Inc. (NTLA)
Event Phase: II
Drug: OTQ923
Indication: Sickle Cell Anemia
Lead Indication: Y
Molecule: Non-Viral Gene Therapy
Target: BCL11A|CRISPR/CRISPR-Cas9
LOA: 34%
Partner Companies: Danaher Corp. (DHR)|Novartis AG (NVS)
Source Link: Clinical Trial of CRISPR-Based Therapy Shows Promise in Sickle Cell Anemia
Novo Nordisk A/S (NVO)
Event Phase: NDA/BLA
Drug: Concizumab
Indication: Hemophilia A and B - General Clotting Products
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Tissue Factor Pathway Inhibitor (TFPI)
LOA: 88%
Partner Companies: N/A
Source Link: Concizumab for Hemophilia A and B with Inhibitors
ADC Therapeutics SA (ADCT)
Event Phase: Approved
Drug: Zynlonta
Indication: Diffuse Large B-Cell Lymphoma (DLBCL) - NHL
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Antibody-drug Conjugate (ADC)|Cluster of Differentiation 19 (CD19)
LOA: 100%
Partner Companies: Mitsubishi Tanabe Pharma Corporation|Overland ADCT BioPharma (CY) Limited|Overland Pharmaceuticals|Swedish Orphan Biovitrum AB (SOBI)
Source Link: ADC Therapeutics Announces Updates on ZYNLONTA LOTIS Clinical Trial Programs
Ultragenyx Pharmaceutical Inc. (RARE)
Event Phase: II
Drug: RGX-111
Indication: Mucopolysaccharidosis I (MPS I; Hurler Syndrome)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Alpha-L-iduronidase|Unsulfated alpha-L-iduronic acid
LOA: 25%
Partner Companies: Regenxbio Inc. (RGNX)
Source Link: Initial Clinical Data of First Pediatric CLN2 Patient Dosed with RGX-181
Travere Therapeutics, Inc. (TVTX)
Event Phase: II
Drug: TVT-058
Indication: Homocystinuria
Lead Indication: Y
Molecule: Protein
Target: Cystathionine beta-synthase (CBS)|Homocysteine
LOA: 27%
Partner Companies: N/A
Source Link: Travere Therapeutics Presents New Data on TVT-058 for Homocystinuria
Sirnaomics, Inc. (2257)
Event Phase: I
Drug: STP707
Indication: Solid Tumors
Lead Indication: N
Molecule: siRNA/RNAi
Target: Cyclooxygenases (COX-1, COX-2, and COX-3)|Transforming Growth Factor-beta (TGF-beta) and Superfamily
LOA: 5%
Partner Companies: N/A
Regenxbio Inc. (RGNX)
Event Phase: IND
Drug: RGX-181
Indication: Neuronal Ceroid Lipofuscinosis (NCL)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Tripeptidyl Peptidase-1 (TTP1)
LOA: N/A
Source Link: Initial Clinical Data of First Pediatric CLN2 Patient Dosed with RGX-181 Presented at SSIEM Annual Symposium
Regenxbio Inc. (RGNX)
Event Phase: III
Drug: RGX-121
Indication: Mucopolysaccharidosis II (MPS II; Hunter Syndrome)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Iduronate-2-Sulfatase |Sulfated alpha-L-iduronic acid
LOA: 58%
Source Link: Poster: Wood: Daily Living Skills on VABS-II in response to RGX-121
Denali Therapeutics Inc. (DNLI)
Event Phase: III
Drug: DNL310
Indication: Mucopolysaccharidosis II (MPS II; Hunter Syndrome)
Lead Indication: Y
Molecule: Protein
Target: Sulfated alpha-L-iduronic acid
LOA: 58%
Source Link: Denali Therapeutics Announces New Interim Data from Phase 1-2 Study of DNL310 (ETV:IDS) in MPS II (Hunter Syndrome)
Praxis Precision Medicines, Inc. (PRAX)
Event Phase: II
Drug: PRAX-944
Indication: Essential Tremor
Lead Indication: Y
Molecule: Small Molecule
Target: Calcium Channel
LOA: 8%
Source Link: Late-Breaking Abstract Booklet: IC23
Orchard Therapeutics Limited (ORTX)
Event Phase: NDA/BLA
Drug: OTL-200
Indication: Metachromatic Leukodystrophy
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Galactosyl-3-sulfate ceramides (sulfatides)|Stem Cells/Other Cell Therapies
LOA: 87%
Partner Companies: AGC Biologics|GSK plc (GSK)
AEON Biopharma, Inc. (AEON)
Event Phase: II
Drug: ABP-450
Indication: Cervical Dystonia
Lead Indication: N
Molecule: Protein
Target: Botulinum toxin|SNARE Proteins (e.g. synaptobrevin, syntaxin, SNAP-25)
LOA: 15%
Neurocrine Biosciences, Inc. (NBIX)
Event Phase: Approved
Drug: Ingrezza
Indication: Tardive Dyskinesia
Lead Indication: Y
Molecule: Small Molecule
Target: Vesicular monamine transporters (VMATs)
LOA: 100%
Partner Companies: Johnson & Johnson (JNJ)|Mitsubishi Tanabe Pharma Corporation
AstraZeneca PLC (AZN)
Event Phase: I
Drug: AZD-8630
Indication: Asthma
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Thymic stromal lymphopoeitin (TSLP)
LOA: 7%
Source Link: Clinical Trial: A Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of AZD-8630
Fresenius SE & Co. KGaA (FSNUY)
Event Phase: Development Outside U.S.
Drug: FKS518
Indication: Osteoporosis / Osteopenia
Lead Indication: N
Molecule: Monoclonal Antibody
Target: RANK Ligand (RANKL)
Clene, Inc. (CLNN)
Event Phase: III
Drug: CNM-Au8
Indication: Amyotrophic Lateral Sclerosis (ALS)
Lead Indication: Y
Molecule: Small Molecule
Target: Myelin
LOA: 41%
FibroGen, Inc. (FGEN)
Event Phase: III
Drug: Pamrevlumab
Indication: Duchenne Muscular Dystrophy (DMD)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Connective Tissue Growth Factor (CTGF)
LOA: 52%
Partner Companies: Bristol Myers Squibb Company (BMY)
Astria Therapeutics, Inc. (ATXS)
Event Phase: I
Drug: STAR-0215
Indication: Hereditary Angioedema (HAE)
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Kinin-Kallikrein System
LOA: 11%
Incyte Corporation (INCY)
Event Phase: II
Drug: Itacitinib
Indication: Myelofibrosis (MF)
Lead Indication: N
Molecule: Small Molecule
Target: JAK/STAT
LOA: 10%
Source Link: A Phase II Study of Itacitinib in Combination With Ruxolitinib for Patients With Myelofibrosis
Financing events
Rebalance Health
Description: Operator of a health and performance lifestyle company intended to optimize health and fitness. The company leverages biotechnology and enhanced delivery to create pure nutraceuticals and peptides, enabling customers to effectively maintain cortisol, testosterone, and human growth hormone (hGH) levels in the body.
Verticals: Life Sciences
Deal Date: August 28, 2023
Deal Type: Early Stage VC
Deal Size: $7.37 million
Investors: Undisclosed investors
Deal Synopsis: The company raised $7.37 million of venture funding from undisclosed investors on August 28, 2023.
Uute Scientific
Description: Developer of health and wellness ingredients intended to eliminate immune-mediated diseases such as diabetes and allergies. The company's ingredients include extracts made from natural substances with diverse microbes from forests and agricultural environments, to be used as an ingredient in ordinary consumer goods, enabling patients to develop their immune systems and decrease the probability of getting such diseases.
Verticals: Life Sciences, LOHAS & Wellness
Deal Date: August 30, 2023
Deal Type: Early Stage VC
Deal Size: EUR 5 million
Deal Synopsis: The company is in the process of raising EUR 5 million of venture funding as of August 30, 2023, putting the company's pre-money valuation at EUR 50 million.
Variational AI
Description: Developer of artificial intelligence platform based on a drug discovery start-up intended to develop novel and selective kinase inhibitors for solid tumor indications, with additional target classes to be added. The company's platform creates novel, potent, selective, safe, and synthesizable small molecules, it's a preclinical therapeutics company and has struck commercial engagements with multiple biopharma and pharma partners, enabling biopharmaceutical companies to perform multi-property molecular optimization to reduce the time to discover drug-like molecules with an adequate probability of success in clinical trials.
Verticals: Artificial Intelligence & Machine Learning, Life Sciences
Deal Date: August 29, 2023
Deal Type: Early Stage VC
XOStem
Description: Developer of a stem cell biotechnology platform designed to treat diabetes. The company's platform researches stem cells and exosomes to create next-generation treatments for type one diabetes and traumatic neurological disorders through engineered exosomes, enabling researchers to develop novel therapies to improve lives.
Verticals: HealthTech, Life Sciences
Deal Date: August 29, 2023
Deal Type: Early Stage VC
Deal Size: $1.44 million
Investors: Undisclosed investors
Deal Synopsis: The company raised $1.44 million of venture funding from undisclosed investors on August 29, 2023.
Antabio
Description: Operator of a private European biopharmaceutical company intended to develop novel and highly differentiated antibacterial treatments. The company's focus is on critical priority pathogens with a particular focus on life-threatening respiratory infections, including carbapenem-resistant nosocomial pneumonia and chronic pulmonary diseases, enabling health professionals to get access to medicinal products that help in curing drug-resistant diseases.
Verticals: HealthTech, Life Sciences, LOHAS & Wellness
Deal Date: August 29, 2023
Deal Type: Later Stage VC
Deal Size: EUR 27.30 million
Investors: European Innovation Council Fund
Deal Synopsis: The company is in the process of raising EUR 25 million of Series B venture funding from European Innovation Council Fund on August 29, 2023.
Cell Microsystems
Description: Developer of biological tools designed for single-cell research. The company's tools can sort and isolate single cells using the array line of consumables under standard culture conditions resulting in unperturbed phenotypes and high viability, enabling scientists to select a cell in real-time and track and trace that cell through imaging, collection, and downstream analysis as well as end-point destructive analysis of single cells for genomics.
Verticals: Life Sciences
Deal Date: August 28, 2023
Deal Type: Later Stage VC
Deal Size: $6.50 million
Investors: Undisclosed investors
Deal Synopsis: The company raised $6.5 million of venture funding from undisclosed investors on August 28, 2023.
QSimulate
Description: Developer of quantitative simulation tools designed to help in quantum molecular simulations in the cloud. The company's cloud-based simulation tools help the chemical and pharmaceutical industries solve the industrial-scale problems they face and the platform automates workflows, freeing computational scientists from mundane tasks to enhance productivity, enabling companies to accelerate their research and development process.
Verticals: HealthTech, Life Sciences, SaaS
Deal Date: August 30, 2023
Deal Type: Later Stage VC
Deal Size: $1.00 million
Investors: Kyoto University Innovation Capital, UTokyo Innovation Platform
Deal Synopsis: The company raised $1 million of venture funding from Kyoto University Innovation Capital and UTokyo Innovation Platform on August 30, 2023. The funds will be used to release the world's first covalent inhibitor design system "QuVa len t(TM) and aims to expand our business in Japan.
Regenerative Medical Solutions
Description: Operator of a biotechnology company intended to provide regenerative medical products to treat diabetes. The company's products include a medical formulation for cell culturing and protocols for growing pancreatic stem cells for the treatment of diabetes, enabling medical institutions to transform stem cells into insulin-producing cells.
Verticals: Life Sciences
Deal Date: August 29, 2023
Deal Type: Later Stage VC
Deal Size: $5.00 million
Investors: Undisclosed investors
Deal Synopsis: The company raised $5 million of venture funding from undisclosed investors on August 29, 2023.
Spiral Therapeutics
Description: Developer of locally delivered drug therapies designed to treat inner ear disorders. The company's therapies target acute and subacute hearing loss and balance disorder indications of neurodegenerative, inflammatory, or vascular origin, enabling patients with hearing disorders to avail themselves of treatments for the prevention and treatment of hearing loss.
Verticals: HealthTech, Life Sciences, LOHAS & Wellness
Deal Date: August 31, 2023
Deal Size: Undisclosed
Investors: Esperante Ventures, Ferring Ventures
Deal Synopsis: The company raised an undisclosed amount of venture funding in a deal led by Esperante Ventures and Ferring Ventures on August 31, 2023. Other undisclosed investors also participated in the round. The funds will be used to accelerate the company's development efforts to treat inner ear disorders, including the collection of additional clinical data for its lead program SPT-2101 for Meniere's Disease, and to advance its hearing loss pipeline.
Stimunity
Description: Operator of a biotechnology company intended to develop STING agonists in cancer. The company's technology provides a biological approach that encapsulates endogenous STING-activating molecules in a virus-like particle, offering medical practitioners a drug that activates the innate immune system and enhances T-cell response against tumor cells with low immunogenicity.
Verticals: Life Sciences, Oncology
Deal Date: August 29, 2023
Deal Type: Later Stage VC
Deal Size: EUR 0.66 million
Investors: Portage Biotech (NAS: PRTG)
Deal Synopsis: The company is in the process of raising EUR 600,000 of Series A venture funding in the form of convertible note from Portage Biotech on August 29, 2023. It plans to close the round by September 1, 2023.
Suono Bio
Description: Developer of therapeutic products designed for the treatment of inflammatory-mediated diseases. The company's platform allows ultra-rapid delivery of therapies and macromolecules across tissues including biologics and nucleic acids that are delivered directly into the gastrointestinal tissues with small molecules, proteins, vaccines, and nucleic acids, enabling patients to recover from gastrointestinal diseases.
Verticals: HealthTech, Life Sciences
Deal Date: August 28, 2023
Deal Type: Later Stage VC
Deal Size: $0.50 million
Investors: Undisclosed investors
Deal Synopsis: The company raised $500,000 of venture funding in the form of convertible debt from undisclosed investors on August 28, 2023.
Twister Biotech
Description: Developer of gene therapies intended to treat rare cancers and autoimmune diseases using a proprietary platform. The company's gene therapies are a result of research, development, and commercialization of supercoiled DNA minicircles for the transfer of DNA into primary and stem cells, enabling researchers to get better transfection efficiencies and long-term effects and also that are easily delivered for safer, effective gene and cell therapies.
Verticals: Life Sciences, Oncology
Deal Date: August 28, 2023
Deal Size: $0.50 million
Investors: Undisclosed investors
Deal Synopsis: The company raised $500,000 of venture funding through a combination of equity and debt from undisclosed investors on August 28, 2023.
Apexigen
Description: Apexigen Inc is a clinical-stage biopharmaceutical company focused on discovering and developing a new generation of antibody therapeutics for oncology, with an emphasis on new immuno-oncology agents designed to harness the patient's immune system to combat and eradicate cancer. The company's pipeline of immuno-oncology therapeutic candidates is led by sotigalimab, which is in clinical development, and also includes several preclinical stage immune-oncology programs.
Verticals: Life Sciences, Oncology
Deal Date: August 29, 2023
Deal Type: Merger/Acquisition
Deal Size: $10.70 million
Investors: Pyxis Oncology (NAS: PYXS)(Lara Sullivan)
Deal Synopsis: The company was acquired by Pyxis Oncology (NAS: PYXS) for $10.7 million on August 29, 2023. This acquisition positions Pyxis Oncology at the forefront of antibody-drug conjugate (ADC) innovation by adding humanized antibody generation to its Flexible Antibody Conjugation Technology (FACT) ADC toolkit.
BianoScience
Description: Developer of DNA and RNA-based drugs designed for drug developers in the pharmaceutical sector. The company offers oligonucleotide production, molecular biology, oligonucleotide analytics, modification and therapeutic RNA prodrug platform development, thereby helping its clients with process development, characterization, validation and scale-ups.
Verticals: Life Sciences, Manufacturing
Deal Date: August 29, 2023
Deal Type: Merger/Acquisition
Deal Size: EUR 10.92 million
Investors: EUROAPI Active Solutions for Health (PAR: EAPI)(Karl Rotthier)
Deal Synopsis: The company reached a definitive agreement to be acquired by EUROAPI Active Solutions for Health (PAR: EAPI) for an estimated EUR 10 million on August 29, 2023. The acquisition will strengthen its CDMO expertise in the high-growth oligonucleotide market.
Embark Biotech
Description: Operator of a biotechnology company intended to identify novel cell surface receptors that physiologically regulate fat tissue calorie-burning and glucose and lipid uptake. The company offers receptors that stimulate energy expenditure without triggering the sympathetic nervous system, enabling users to address clinical unmet needs within the metabolic diseases sphere.
Verticals: HealthTech, Life Sciences
Deal Date: August 30, 2023
Deal Type: Merger/Acquisition
Deal Size: EUR 16.37 million
Investors: Novo Nordisk (CSE: NOVO B)
Deal Synopsis: The company was acquired by Novo Nordisk (CSE: NOVO B) for EUR 15 million on August 30, 2023.
Small Pharma (TSX: DMT)
Description: Small Pharma Inc is a neuropharmaceutical company. It is committed towards the development of effective therapeutic treatments for mental health disorders. Small Pharma is developing N,N-dimethyltryptamine and a pipeline of novel patent-protected deuterium-enriched tryptamine compounds in combination with psychotherapy as potential rapid onset, sustained treatments for depression and other mental health disorders. It has one operating segment, research and development ("R&D") of psychedelic and nonpsychedelic medicine.
Verticals: Life Sciences
Deal Date: August 28, 2023
Deal Type: Merger/Acquisition
Investors: Cybin (NEOE: CYBN)(Douglas Drysdale)
Deal Synopsis: The company reached a definitive agreement to be acquired by Cybin (NEOE: CYBN) for an undisclosed amount on August 28, 2023. The synergy of Cybin's and the company's development programs, intellectual property and robust datasets enhances our leadership and expertise in developing potentially best-in-class, optimized psychedelic therapeutics and positions the combined company to generate long-term value for all stakeholders.
Johnson County Clin-Trials
Description: Provider of clinical research and contract research services intended for pharmaceutical and biotechnology companies. The company specializes in offering Phase I to IV outpatient and inpatient clinical trials, encompassing healthy volunteers and special populations, patient engagement and data integrity, thereby enabling its sponsors in the development and approval of new or improved drugs and vaccines brought to market.
Verticals: Life Sciences
Deal Date: August 28, 2023
Deal Type: PE Growth/Expansion
Investors: FFL Partners(Karen Winterhof)
Deal Synopsis: The company received an undisclosed amount of development capital from FFL Partners on August 28, 2023. As a result of the transaction, the company was recapitalized.
Inhibrx (NAS: INBX)
Description: Inhibrx Inc is a clinical-stage biotechnology company focused on developing a pipeline of novel biologic therapeutic candidates. It combines an understanding of target biology with protein engineering, proprietary discovery technologies, and an integrative approach to research and development to design differentiated therapeutic candidates. The company has four programs in clinical trials, of which three of the programs are for the treatment of various cancers, and one for the treatment of Alpha-1 Antitrypsin Deficiency.
Verticals: Life Sciences, Oncology
Deal Date: August 29, 2023
Deal Type: PIPE
Deal Size: $200.00 million
Investors: Perceptive Advisors, RA Capital Management, TCG Crossover Management, Viking Global Investors
Deal Synopsis: The company (NAS: INBX) is in talks to receive approximately $200 million of development capital from TCG Crossover Management, RA Capital Management, Viking Global Investors, and Perceptive Advisors through a private placement as of August 29, 2023.
TC Biopharm (NAS: TCBP)
Description: TC BioPharm (Holdings) PLC is a clinical-stage biopharmaceutical company focused on developing novel immunotherapy products that are based on its proprietary allogeneic gamma delta T (abbreviated as GD-T) cell platform.
Verticals: Life Sciences, Oncology
Deal Date: August 28, 2023
Deal Type: Public Investment 2nd Offering
Deal Size: $5.00 million
Deal Synopsis: The company filed for a second public offering on the Nasdaq stock exchange under the ticker symbol of TCBP on August 28, 2023. They intend to sell 9,900,990 ADS or American Depository Shares at a price of $0.505 per ADS or American Depository Shares. The expected offering amount is $5 million.
TransCode Therapeutics (NAS: RNAZ)
Description: TransCode Therapeutics Inc is an RNA oncology company. It is created to defeat cancer through the intelligent design and effective delivery of RNA therapeutics. The company's therapeutic candidate, TTX-MC138, is focused on treating metastatic cancer, which has the potential to produce regression without recurrence in a range of cancers, including breast, pancreatic, ovarian, and colon cancer, glioblastomas, and others.
Verticals: Life Sciences, Oncology
Deal Date: August 29, 2023
Deal Type: Public Investment 2nd Offering
Deal Synopsis: The company filed for a second public offering on the Nasda stock exchange under the ticker symbol of RNAZ on August 29, 2023.
Glycoscience
Description: Developer of natural insect repellents designed for consumers. The company's technology allows adding sugars to natural molecules, which enhances their activity, safety, absorption and release time, enabling users to get an alternative to current insect repellents, which are often synthetic and can have harmful side effects.
Verticals: Life Sciences
Deal Date: August 28, 2023
Deal Type: Seed Round
Deal Size: EUR 2.08 million
Investors: Undisclosed investors
Deal Synopsis: The company raised EUR 1.9 million of seed funding from undisclosed investors on August 28, 2023.
NED Medical
Description: Developer of medical technology designed to provide treatment for liver cancer. The company's technology helps to transform oncology embolization from a palliative to a curative treatment while reducing the debilitating side effects and toxicities, enabling healthcare professionals to provide better treatment to cancer patients.
Verticals: Life Sciences, LOHAS & Wellness, Oncology
Deal Date: August 28, 2023
Deal Type: Seed Round
Deal Size: $0.50 million
Investors: Undisclosed investors
Deal Synopsis: The company raised $500,000 of seed funding from undisclosed investors on August 28, 2023.
Superluminal Medicines
Description: Developer of generative biology medicine technology intended to accelerate new possibilities in drug discovery and development. The company creates candidate-ready compounds using a comprehensive combination of deep biology and chemistry expertise, machine learning, and proprietary big data infrastructure, enabling the pharmaceutical industry to accelerate drug discovery and increase the probability of success in small molecule drug development.
Verticals: Artificial Intelligence & Machine Learning, Big Data, Life Sciences
Deal Date: August 28, 2023
Deal Type: Seed Round
Deal Size: $33.00 million
Investors: Gaingels, Insight Partners(Dylan Morris), Nvidia (NAS: NVDA), RA Capital Management(Andrew Levin)
Deal Synopsis: The company raised $33 million through a combination of Seed-1 and Seed-2 funding in a deal led by RA Capital Management on August 28, 2023, putting the company's pre-money valuation at $8 million. Nvidia, Insight Partners, and Gaingels also participated in the round. The funds will be used to progress the company's pipeline of small molecule drug discovery programs initially focused on high-value G protein-coupled receptor targets.
Reduction in force (RIF)
August 31 - Sage Therapeutics: After alluding to potential layoffs in August following an FDA rejection, Sage is sending 40% of its team out the door. Alongside a pipeline trim, the biotech is also saying goodbye to long-standing Chief Scientific Officer Al Robichaud, Ph.D., along with Chief Development Officer Jim Doherty and Mark Pollack, senior vice president of medical affairs. Story
August 30 - Poxel: French biotech Poxel expanded on the previously disclosed layoffs announced at the end of 2022 in its latest earnings report. The company says it now has 16 employees compared to 37 in December 2022, a 57% reduction. Release
August 29 - Notch Therapeutics: The cell therapy biotech is closing one of its three centers and is working to find positions for its displaced staff at other companies in the industry. The shutdown will impact about 25 people, with some of those team members being offered relocation spots at the company's Toronto or Seattle centers. Story
Disease of the week
Narcolepsy with Cataplexy is a complex neurological disorder that affects sleep-wake regulation and muscle control. It falls under the broader category of sleep disorders and is characterized by the following key features:
Excessive Daytime Sleepiness (EDS): People with narcolepsy with cataplexy experience an overwhelming and persistent need to sleep during the daytime. They often have difficulty staying awake and alert, which can lead to sudden and uncontrollable sleep attacks. These sleep attacks can occur during various activities, such as eating, talking, or working.
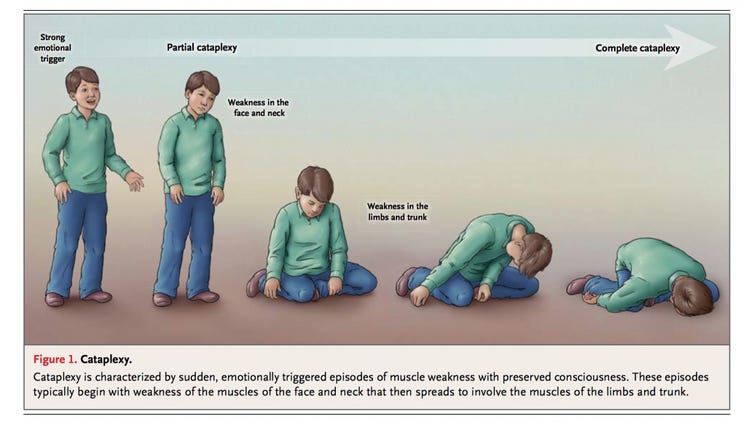
Cataplexy: This symptom is unique to narcolepsy with cataplexy. Cataplexy involves a sudden loss of muscle tone or muscle weakness triggered by strong emotions, particularly positive ones like laughter, surprise, or excitement. The person remains conscious during these episodes but might experience varying degrees of muscle weakness or paralysis. This can range from minor facial drooping to complete muscle collapse.
Sleep Paralysis: Individuals with narcolepsy can experience episodes of sleep paralysis, which is the temporary inability to move or speak while falling asleep or waking up. These episodes can be frightening and are often accompanied by vivid hallucinations.
Hypnagogic and Hypnopompic Hallucinations: These hallucinations occur when falling asleep (hypnagogic) or waking up (hypnopompic). They can involve sensory experiences like seeing, hearing, or feeling things that aren't actually present.
Fragmented Nighttime Sleep: People with narcolepsy often experience disrupted nighttime sleep with frequent awakenings. This contributes to their excessive daytime sleepiness.
Prevalence: Narcolepsy with cataplexy is relatively rare, affecting an estimated 1 in 2,000 individuals. It can develop at any age, but it often becomes noticeable during adolescence or young adulthood.
Causes: The exact cause of narcolepsy with cataplexy is not fully understood. However, it is associated with a deficiency of the neurotransmitter hypocretin (also known as orexin), which plays a crucial role in regulating wakefulness and sleep. This deficiency is thought to result from an autoimmune reaction in which the body's immune system attacks the cells that produce hypocretin.
Diagnosis: Diagnosing narcolepsy with cataplexy involves a combination of clinical evaluation, medical history, and sleep studies (polysomnography and multiple sleep latency tests). These tests help rule out other sleep disorders and confirm the presence of the characteristic symptoms.
Treatment: While there is no cure for narcolepsy with cataplexy, various treatment approaches can help manage the symptoms and improve the quality of life:
Stimulant Medications: These medications, like modafinil or amphetamines, help promote wakefulness and reduce daytime sleepiness.
Sodium Oxybate: This medication can improve nighttime sleep and reduce cataplexy episodes.
Antidepressants: Certain antidepressant medications, particularly selective serotonin and norepinephrine reuptake inhibitors (SSNRIs), can help control cataplexy and improve mood.
Lifestyle Adjustments: Establishing regular sleep routines, managing stress, and avoiding excessive caffeine and alcohol intake can contribute to better symptom management.
Scheduled Naps: Short, planned naps during the day can help reduce the frequency of sudden sleep attacks.
Supportive Therapies: Cognitive behavioral therapy (CBT) can help individuals cope with the emotional and psychological aspects of living with narcolepsy.
What I’ve read this week
Text on why pain is the most unmet need
Strategic implications for biopharma following the IRA (From:
)