Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
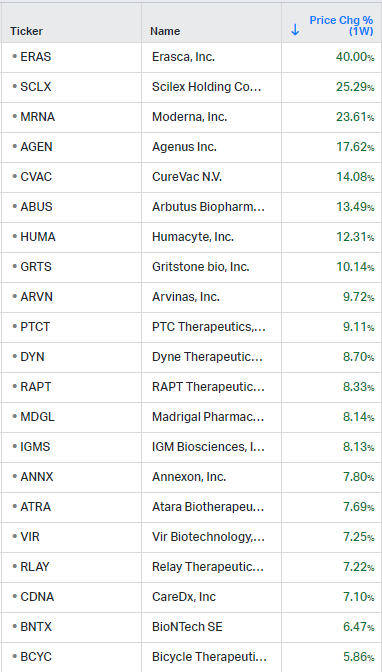
How the market performed this week
ETFs
Gene Therapy
RNA Therapy
Big Pharma
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Clinuvel Pharmaceuticals Limited (CLVLY)
Event Phase: Approved
Drug: Scenesse
Indication: Porphyria
Target: Melanocortin (MC) receptors
LOA: 100%
AbbVie Inc. (ABBV)
Event Phase: II
Drug: NX-13
Indication: Ulcerative Colitis (UC)
Target: Mitochondria
LOA: 19%
AbbVie Inc. (ABBV)
Event Phase: I
Drug: NX-13
Indication: Crohn's Disease
Target: Mitochondria
LOA: 11%
AbbVie Inc. (ABBV)
Event Phase: Preclinical
Drug: LABP-66
Indication: Multiple Sclerosis (MS)
Target: Mitochondria
AbbVie Inc. (ABBV)
Event Phase: Preclinical
Drug: LABP-66
Indication: Alzheimer's Disease (AD)
Target: Mitochondria
AbbVie Inc. (ABBV)
Event Phase: Preclinical
Drug: LABP-69
Indication: Rheumatoid Arthritis (RA)
Target: Plexin Domain Containing 2 (PLXDC2)
AbbVie Inc. (ABBV)
Event Phase: Preclinical
Drug: LABP-73
Indication: Asthma
Target: Mitochondria
Active Biotech AB (ACTI)
Event Phase: II
Drug: TASQ
Indication: Multiple Myeloma (MM)
Target: Angiogenesis, Migration inhibitory factor-related protein 14 (MRP14)/S100A9
LOA: 11%
Acorda Therapeutics, Inc. (ACOR)
Event Phase: Approved
Drug: Inbrija
Indication: Parkinson's Disease (PD)
Target: Dopamine Receptor - Unspecified
LOA: 100%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1107
Indication: Triple-Negative Breast Cancer (TNBC)
Target: Tyrosine Kinases
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1107
Indication: Uterine (Endometrial) Cancer
Target: Tyrosine Kinases
LOA: 11%
Genmab A/S (GMAB)
Event Phase: Development Outside U.S.
Drug: PRO1102
Indication: Solid Tumors
Target: HER2/neu or ErbB-2
Genmab A/S (GMAB)
Event Phase: Development Outside U.S.
Drug: PRO1106
Indication: Solid Tumors
Target: Slitrk6
Genmab A/S (GMAB)
Event Phase: Development Outside U.S.
Drug: PRO1286
Indication: Solid Tumors
Target: EGFR (Epidermal Growth Factor Receptor)
BeiGene, Ltd. (BGNE)
Event Phase: III
Drug: Tevimbra
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Immune System, Programmed death-1 receptor (PD-1)
LOA: 44%
Dr. Reddy's Laboratories Ltd. (RDY)
Event Phase: Development Outside U.S.
Drug: AVT03
Indication: Bone Complications (Including Bone Metastases)
Target: RANK Ligand (RANKL)
Qurient Co., Ltd. (115180)
Event Phase: II
Drug: Q901
Indication: Solid Tumors
Target: Cyclin Dependent Kinase 7 (CDK-7)
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1184
Indication: Solid Tumors
Target: Folate Receptor (FOLR1)
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1160
Indication: Solid Tumors
Target: Cluster of Differentiation 70 (CD70)
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1107
Indication: Bladder Cancer
Target: Tyrosine Kinases
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1107
Indication: Esophageal Cancer
Target: Tyrosine Kinases
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1107
Indication: Gastric Cancer
Target: Tyrosine Kinases
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1107
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Tyrosine Kinases
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1107
Indication: Ovarian Cancer
Target: Tyrosine Kinases
LOA: 11%
Genmab A/S (GMAB)
Event Phase: II
Drug: PRO1107
Indication: Solid Tumors
Target: Tyrosine Kinases
LOA: 11%
Clinical trials (LOA=likelihood of approval)
Evaxion Biotech A/S (EVAX)
Drug: EVX-01
Indication: Melanoma
Target: Immune System, Tumor Cells
Event Phase: Phase IIb (w/Keytruda)
LOA: -
Source Link: Link
Affimed N.V. (AFMD)
Drug: AFM24
Indication: Solid Tumors
Target: Cluster of Differentiation 16 (CD16), EGFR
Event Phase: Phase I/IIa (w/Atezolizumab)
LOA: 11%
Source Link: Link
Bio-Path Holdings, Inc. (BPTH)
Drug: Prexigebersen
Indication: Acute Myelogenous Leukemia (AML)
Target: Growth factor receptor bound protein 2 (Grb-2)
Event Phase: Phase II (w/Decitabine)
LOA: 12%
Source Link: Link
FibroGen, Inc. (FGEN)
Drug: FG-3246
Indication: Prostate Cancer
Target: Cluster of Differentiation 46 (CD46)
Event Phase: Phase Ib/II (w/ Enzalutamide)
LOA: 11%
Source Link: Link
Iovance Biotherapeutics, Inc. (IOVA)
Drug: Amtagvi
Indication: Melanoma
Target: Immune System, Stem Cells/Other Cell Therapies, T lymphocytes
Event Phase: Approved (Phase II)
LOA: 100%
Source Link: Link
Candel Therapeutics, Inc. (CADL)
Drug: CAN-2409
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Thymidine Kinase, Tumor Cells
Event Phase: Phase II
LOA: 11%
Source Link: Link
Alligator Bioscience AB (ATORX)
Drug: ADC-1013
Indication: Pancreatic Cancer
Target: Cluster of Differentiation 40 (CD40), Immune System
Event Phase: Phase Ib/II (EU)
LOA: -
Source Link: Link
Pfizer Inc. (PFE)
Drug: Tivdak
Indication: Solid Tumors
Target: Tissue Factor
Event Phase: Phase II
LOA: 11%
Source Link: Link
Genmab A/S (GMAB)
Drug: Epkinly
Indication: Follicular Lymphoma (FL)
Target: Cluster of Differentiation 20 (CD20), Cluster of Differentiation 3 (CD3)
Event Phase: NDA/BLA (Phase I/II)
LOA: 92%
Source Link: Link
Novartis AG (NVS)
Drug: ARV-766
Indication: Prostate Cancer
Target: Androgen receptors
Event Phase: Phase I/II (w/Abiraterone)
LOA: 11%
Source Link: Link
Johnson & Johnson (JNJ)
Drug: Rybrevant
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: EGFR, c-Met (HGFR)
Event Phase: Approved (Phase III)
LOA: 100%
Source Link: Link
Novartis AG (NVS)
Drug: DFF332
Indication: Renal Cell Cancer (RCC)
Target: Hypoxia Inducible Factor-2 Alpha (HIF-2a)
Event Phase: Phase I
LOA: 5%
Source Link: Link
Agenus Inc. (AGEN)
Drug: Botensilimab
Indication: Colorectal Cancer (CRC)
Target: Cytotoxic T-Lymphocyte Antigen 4 (CTLA4)
Event Phase: Phase Ia/Ib (PK/PD Study)
LOA: 11%
Source Link: Link
Bristol Myers Squibb Company (BMY)
Drug: Onureg
Indication: Myelodysplastic Syndrome (MDS)
Target: DNA Methyltransferase (DNMT)
Event Phase: Phase II/III
LOA: 37%
Source Link: Link
Merus N.V. (MRUS)
Drug: MCLA-145
Indication: Solid Tumors
Target: Cluster of Differentiation 137 (CD 137), Immune System, PD-L1
Event Phase: Phase I
LOA: 5%
Source Link: Link
Eli Lilly and Company (LLY)
Drug: Verzenio
Indication: Prostate Cancer
Target: Cyclin Dependent Kinase 4 (CDK-4), Cyclin Dependent Kinase 6 (CDK-6)
Event Phase: Phase II/III
LOA: 11%
Source Link: Link
Karyopharm Therapeutics (KPTI)
Drug: Xpovio
Indication: Peripheral T-Cell Lymphoma (PTCL) - NHL
Target: Exportin-1/CRM1/XPO1, Immune System, Interferon-beta (IFNb), SARS-CoV-2
Event Phase: Phase Ib
LOA: -
Source Link: Link
AstraZeneca PLC (AZN)
Drug: ATG-017
Indication: Solid Tumors
Target: MAPK/ERK, MAPK1/ERK2, MAPK3/ERK1
Event Phase: Phase I
LOA: 5%
Source Link: Link
Blueprint Medicines Corporation (BPMC)
Drug: BLU-222
Indication: HR+/HER2- Breast Cancer
Target: Cyclin Dependent Kinase 2 (CDK-2)
Event Phase: Phase I/II
LOA: 11%
Source Link: Link
Coherus BioSciences, Inc.
Ticker: CHRS
Phase: II
Trial: Phase I/II - Dose Escalation/Expansion
Drug: CHS-114
Indication: Solid Tumors
Target: Chemokine Receptor 8 (CCR8)
Response Rate: 11%
Link: News Release
Bristol Myers Squibb Company
Ticker: BMY
Phase: Approved
Trial: Phase III - TRANSFORM
Drug: Breyanzi
Indication: Diffuse Large B-Cell Lymphoma (DLBCL) - NHL
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), Cluster of Differentiation 19 (CD19), Immune System, T lymphocytes
Response Rate: 100%
Link: News Release
Cantargia AB
Ticker: CANTA
Phase: II
Trial: Phase I/IIa - CANFOUR (BeNeLux/Scandinavia), Preclinical Studies
Drug: CAN-04
Indication: Pancreatic Cancer
Target: IL-1 Receptor (IL-1R)
Response Rate: 11%
Link: News Release
AADi Bioscience, Inc.
Ticker: AADI
Phase: Preclinical
Trial: Preclinical Studies
Drug: Fyarro
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Mammalian Target of Rapamycin (mTOR)/mTORC
Link: News Release
Azitra Inc.
Ticker: AZTR
Phase: Preclinical
Trial: Preclinical Studies
Drug: ATR-04
Indication: Staphylococcal Vaccines and Other Staphylococcus-Specific Agents (Antibacterial)
Target: Microbiota (flora, microbiome) excluding the digestive tract
Link: News Release
Roche Holding AG
Ticker: RHHBY
Phase: III
Trial: Phase I/II - NP30179 (w/Gazyva)
Drug: Columvi
Indication: Mantle Cell Lymphoma - NHL
Target: Cluster of Differentiation 20 (CD20), Cluster of Differentiation 3 (CD3), Immune System
Response Rate: 44%
Link: News Release
Bristol Myers Squibb Company
Ticker: BMY
Phase: II
Trial: Phase I - POC - w/BMC128 (Israel)
Drug: Opdivo
Indication: Solid Tumors
Target: Immune System, Programmed death-1 receptor (PD-1)
Response Rate: 11%
Link: News Release
Supernus Pharmaceuticals, Inc.
Ticker: SUPN
Phase: II
Trial: Phase IIa - RENAISSANCE
Drug: SPN-817
Indication: Partial / Focal Seizures (Epilepsy)
Target: Cholinesterases
Response Rate: 12%
Link: News Release
HOOKIPA Pharma Inc.
Ticker: HOOK
Phase: II
Trial: Phase I/II - HPV-Positive Cancers (HB-201/HB-202)
Drug: HB-200
Indication: Head and Neck Cancer
Target: Human Papillomavirus (HPV), Immune System
Response Rate: 12%
Link: News Release
Cogent Biosciences, Inc.
Ticker: COGT
Phase: III
Trial: Phase III - PEAK
Drug: Bezuclastinib
Indication: Gastrointestinal Stromal Tumor (GIST)
Target: KIT/c-KIT
Response Rate: 45%
Link: News Release
Kronos Bio, Inc.
Ticker: KRON
Phase: II
Trial: Phase I/II - MYC-Amplified
Drug: KB-0742
Indication: Solid Tumors
Target: Cyclin Dependent Kinase 9 (CDK-9)
Response Rate: 11%
Link: News Release
Compass Therapeutics Inc
Ticker: CMPX
Phase: I
Trial: Phase Ib - CTX-471-001
Drug: CTX-471
Indication: Solid Tumors
Target: Cluster of Differentiation 137 (CD 137), Immune System
Response Rate: 11%
Link: News Release
Amgen Inc.
Ticker: AMGN
Phase: III
Trial: Phase III - THOR
Drug: Lumakras
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS)
Link: News Release
Financing events
AltruBio
Description: Develops antibody therapeutics for cancer and immune-related diseases, focusing on antibody-drug conjugates and targeted tumor indications.
Verticals: Life Sciences, Oncology
Deal Date: May 21, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $225 million in Series B funding led by BVF Partners to support Phase 2a and 2b clinical trials of ALTB-268 in ulcerative colitis.
Investors: aMoon Fund, Blackstone, BVF Partners, Cormorant Asset Management, Delos Capital Partners, RA Capital Management, Soleus Capital
Deal Size: $225 million
Amplexd Therapeutics
Description: Provides early cervical cancer therapeutics, offering non-invasive treatments as alternatives to surgeries for dysplasia.
Verticals: Life Sciences, Oncology
Deal Date: May 19, 2024
Deal Type: Early Stage VC
Deal Synopsis: Raised $2 million to finalize development and IND submissions for first-in-human clinical trials.
Investors: Undisclosed
Deal Size: $2 million
Better Therapeutics (PINX: BTTX)
Description: Develops prescription digital therapeutics for cardiometabolic diseases using cognitive behavioural therapy.
Verticals: AI & Machine Learning, Digital Health, Life Sciences, LOHAS & Wellness, Mobile, TMT
Deal Date: May 22, 2024
Deal Type: Merger/Acquisition
Deal Synopsis: To be acquired by Click Therapeutics for an undisclosed amount.
Investors: Click Therapeutics
Bicycle Therapeutics (DUS: 50BA)
Description: Develops a novel class of medicines called Bicycles, focusing on oncology indications.
Verticals: Life Sciences, Oncology
Deal Date: May 23, 2024
Deal Type: PIPE
Deal Synopsis: In talks to receive $555.49 million in development capital from various investors for proprietary pipeline development.
Investors: Deep Track Capital, EcoR1 Capital, Fairmount Partners, Forbion, Perceptive Advisors, RA Capital Management
Deal Size: $555.50 million
Captain T Cell
Description: Develops enhanced T-cell therapies for solid tumors.
Verticals: Life Sciences, Oncology
Deal Date: May 22, 2024
Deal Type: Seed Round
Deal Synopsis: Raised EUR 8.5 million to advance lead program and novel allogeneic platform.
Investors: Brandenburg Kapital, HIL-INVENT, i&i Biotech Fund
Deal Size: $9.16 million
CUTISS
Description: Develops bio-engineered skin grafts for large skin defects.
Verticals: HealthTech, Life Sciences
Deal Date: May 21, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised CHF 25.31 million to develop personalized skin therapy denovoSkin.
Investors: Cherry Bay Capital Group, Giuliani
Deal Size: $27.83 million
Dyne Therapeutics (NAS: DYN)
Description: Focuses on therapeutics for genetically driven muscle diseases.
Verticals: HealthTech, Life Sciences
Deal Date: May 22, 2024
Deal Type: Public Investment 2nd Offering
Deal Synopsis: Raised $325.5 million through public offering on Nasdaq.
Investors: Public Offering
Deal Size: $325.50 million
En Carta Diagnostics
Description: Develops molecular diagnostics platform using synthetic biology.
Verticals: HealthTech, Life Sciences
Deal Date: May 22, 2024
Deal Type: Early Stage VC
Deal Synopsis: Raised EUR 1.5 million to develop a Lyme disease diagnostic kit.
Investors: CentraleSupélec, CVS Health
Deal Size: $1.62 million
Enrich Biosystems
Description: Develops single-cell isolation technology for biomedical research.
Verticals: HealthTech, Life Sciences, LOHAS & Wellness
Deal Date: May 20, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $2.99 million, including $750,000 in convertible debt.
Investors: Undisclosed
Deal Size: $2.99 million
Fiberwood
Description: Manufactures sustainable wood-based thermal insulation boards.
Verticals: Life Sciences, LOHAS & Wellness, Manufacturing
Deal Date: May 23, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised EUR 7.7 million for ecological insulation and packaging development.
Investors: Metsä Spring, Stephen Industries
Deal Size: $8.29 million
Foghorn Therapeutics (NAS: FHTX)
Description: Develops medicines targeting the chromatin regulatory system for cancer treatment.
Verticals: Life Sciences, Oncology
Deal Date: May 20, 2024
Deal Type: PIPE
Deal Synopsis: In talks to receive $70.21 million for continued development of its pipeline.
Investors: Undisclosed
Deal Size: $70.21 million
Grey Wolf Therapeutics
Description: Develops oncology therapies focusing on immune-oncology by altering tumor cell antigens.
Verticals: Life Sciences, Oncology
Deal Date: May 23, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised GBP 99 million to develop immuno-oncology approaches.
Investors: Pfizer Ventures, Intermediate Capital Group, Earlybird Venture Capital, Andera Partners, British Patient Capital, Canaan Partners, Oxford Science Enterprises
Deal Size: $124.30 million
GT Biopharma (NAS: GTBP)
Description: Focuses on immuno-oncology products for cancer treatment using TriKE platforms.
Verticals: Life Sciences, Oncology
Deal Date: May 23, 2024
Deal Type: PIPE
Deal Synopsis: Received $3.22 million for general corporate purposes.
Investors: Undisclosed
Deal Size: $3.22 million
HI-Bio
Description: Develops precision medicines for autoimmune and inflammatory diseases.
Verticals: Life Sciences
Deal Date: May 22, 2024
Deal Type: Merger/Acquisition
Deal Synopsis: To be acquired by Biogen for $1.15 billion.
Investors: Biogen
Deal Size: $1.15 billion
InBrain Pharma
Description: Develops brain infusion technology for neurodegenerative disease treatment.
Verticals: HealthTech, Life Sciences, TMT
Deal Date: May 22, 2024
Deal Type: Seed Round
Deal Synopsis: Raised EUR 3.2 million from undisclosed investors.
Investors: Undisclosed
Deal Size: $3.45 million
InDex Pharmaceuticals (STO: INDEX)
Description: Focuses on treatments for immunological diseases, including ulcerative colitis.
Verticals: Life Sciences, Oncology
Deal Date: May 20, 2024
Deal Type: Merger/Acquisition
Deal Synopsis: To be acquired by Flerie through a reverse merger.
Investors: Flerie
Reduction in force (RIF)
May 21 - Lyra Therapeutics: The biotech is laying off 87 employees, including Chief Technology Officer John Bishop, Ph.D. The layoffs are effective today for 80 people and on June 20 for the remaining seven. Story
May 21 - Exscientia: The AI drug hunter is initiating “efficiency measures” to save cash, which includes a workforce reduction expected to impact between 20% and 25% of staff by the end of this year. Story
May 20 - BIO: The biotech advocacy organization is implementing a "strategic plan" that includes laying off 30 people, according to an emailed statement from President and CEO John Crowley. The restructure is designed to "bring stronger focus and greater impact in everything we do," Crowley said.
Disease of the week
Von Hippel-Lindau (VHL) disease is a rare genetic disorder characterized by the development of tumors and cysts in various parts of the body. It's caused by mutations in the VHL gene, which plays a crucial role in regulating cell growth and division. This gene is located on the short arm of chromosome 3.
Here's a breakdown of key aspects of Von Hippel-Lindau disease:
Inheritance: VHL disease follows an autosomal dominant pattern of inheritance, meaning that a person only needs to inherit one mutated copy of the VHL gene from either parent to develop the disorder. If a parent has VHL, each of their children has a 50% chance of inheriting the mutated gene.
Clinical Features: The clinical manifestations of VHL disease can vary widely among affected individuals, even within the same family. The most common features include:
Hemangioblastomas: These are benign tumors that often occur in the brain, spinal cord, and retina. They can cause symptoms such as headaches, vision problems, and neurological deficits.
Renal cell carcinoma (RCC): Individuals with VHL have an increased risk of developing kidney cancer, particularly clear cell renal cell carcinoma.
Pheochromocytomas and paragangliomas: These tumors arise from specialized cells of the adrenal glands (pheochromocytomas) or from nerve cells associated with the sympathetic nervous system (paragangliomas). They can cause symptoms related to excess catecholamine production, such as high blood pressure, palpitations, and sweating.
Pancreatic neuroendocrine tumors (PNETs): These tumors can develop in the pancreas and may produce hormones that cause various symptoms, depending on the type of hormone produced.
Cysts and tumors in other organs: VHL disease can also lead to the formation of cysts and tumors in the kidneys, pancreas, adrenal glands, epididymis (in males), and other organs.
Diagnosis: The diagnosis of VHL disease is based on clinical features, family history, and genetic testing to identify mutations in the VHL gene. Imaging studies such as MRI or CT scans are often used to detect and monitor tumors and cysts associated with the condition.
Management and Treatment: There is currently no cure for VHL disease, but treatment focuses on managing symptoms and reducing the risk of complications. This may include surveillance through regular imaging studies to monitor tumor growth, surgical removal of tumors or cysts that cause symptoms or complications, and targeted therapies for certain types of tumors, such as RCC.
Genetic Counseling: Genetic counseling is recommended for individuals with VHL disease and their family members to understand the inheritance pattern, the risk of passing the condition to future generations, and the options for genetic testing and family planning.
What I’ve read this week
*Click on the pic to read*