Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
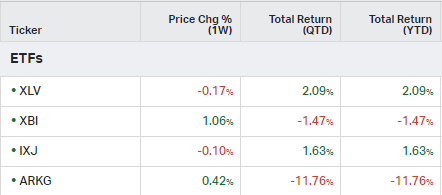
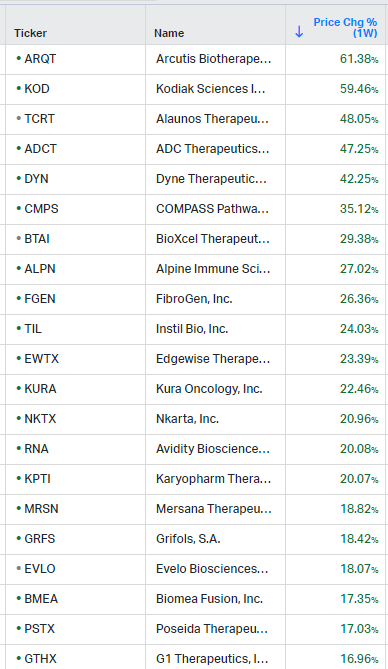
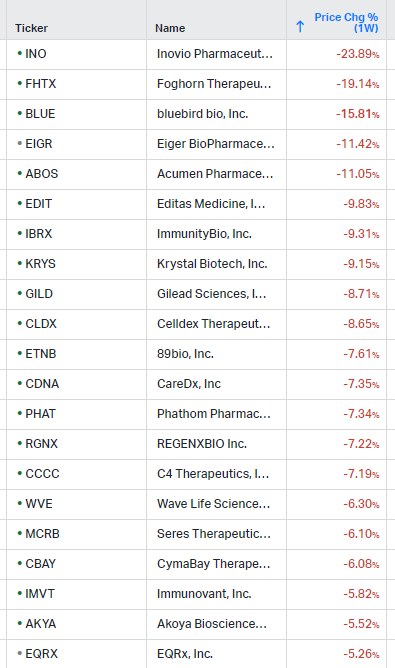
How the market performed this week
ETFs
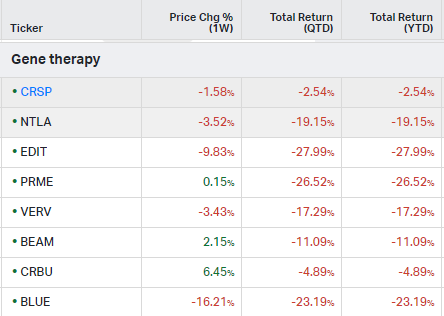
Gene Therapy
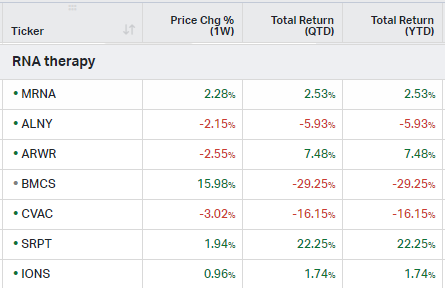
RNA Therapy
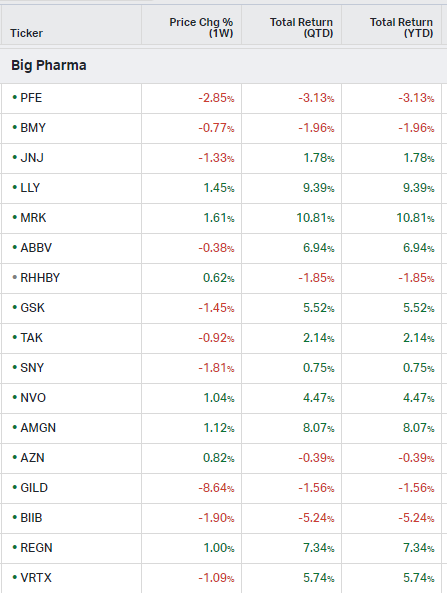
Big Pharma
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
TRACON Pharmaceuticals Inc.
Symbol: TCON
Event Phase: II
Drug: Envafolimab
Indication: Soft Tissue Sarcoma - General
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Immune System, Programmed death-ligand 1 (PD-L1)
LOA: 11%
Partner Companies: 3D Medicines Inc. (1244), Alphamab Oncology (9966), Ascletis Pharma, Inc. (1672), Glenmark Pharmaceuticals Limited (GNP), Simcere Pharmaceutical Group (2096)
Source Link: PR Newswire
Travere Therapeutics, Inc.
Symbol: TVTX
Event Phase: Approved
Drug: Filspari
Indication: Immunoglobulin A (IgA) Nephropathy (Berger's Disease)
Lead Indication: Y
Molecule: Small Molecule
Target: Angiotensin II Receptor Type 1 (AT1), Endothelin Receptor Type A (EDNRA)
LOA: 100%
Partner Companies: Bristol Myers Squibb Company (BMY), CSL Vifor, Ligand Pharmaceuticals, Inc. (LGND), Renalys Pharma
Source Link: GlobeNewswire
Kyowa Kirin Co., Ltd.
Symbol: 4151:JP
Event Phase: Development Outside U.S.
Drug: Strimvelis
Indication: Primary Immunodeficiencies
Molecule: Viral Gene Therapy
Target: Adenosine Deaminase (ADA)
Partner Companies: AGC Biologics
Source Link: Business Wire
[Continued for Kyowa Kirin Co., Ltd.]
Pharming Group N.V.
Symbol: PHAR
Event Phase: Preclinical
Drug: OTL-105
Indication: Hereditary Angioedema (HAE)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Stem Cells/Other Cell Therapies
LOA: Kyowa Kirin Co., Ltd. (4151:JP)
Source Link: Business Wire
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: Approved
Drug: Krazati
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: N
Molecule: Small Molecule
Target: KRas
LOA: 100%
Partner Companies: Zai Lab Ltd. (ZLAB)
Source Link: Business Wire
[Continued for Bristol Myers Squibb Company.]
ORIC Pharmaceuticals, Inc.
Symbol: ORIC
Event Phase: I
Drug: ORIC-944
Indication: Prostate Cancer
Lead Indication: Y
Molecule: Small Molecule
Target: EZH1/2
LOA: 5%
Partner Companies: Bristol Myers Squibb Company (BMY)
Source Link: Business Wire
Bayer AG
Symbol: BAYN
Event Phase: Preclinical
Drug: OpCT-001
Indication: Other Retinopathy (Ophthalmology)
Lead Indication: N
Molecule: Cellular
Target: Immune System, Stem Cells/Other Cell Therapies
Partner Companies: FUJIFILM Holdings Corp. (TYO:4901), Opsis Therapeutics, LLC
Source Link: PR Newswire
Inhibrx, Inc.
Symbol: INBX
Event Phase: II
Drug: INBRX-101
Indication: Alpha-1 Antitrypsin Deficiency (A1AD or AATD)
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Alpha-1-antitrypsin (A1AT)
LOA: 25%
Source Link: PR Newswire
Sandoz International GmbH
Symbol: SDZ
Event Phase: Approved
Drug: Cimerli
Indication: Myopic Macular Degeneration (MMD)/Pathological Myopia (Ophthalmology)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: VEGF (Vascular endothelial growth factor)
LOA: 100%
Partner Companies: Bioeq IP AG, Coherus BioSciences, Inc. (CHRS), Formycon AG (FYB), Teva Pharmaceutical Industries Ltd. (TEVA)
Source Link: GlobeNewswire
AC Immune SA
Symbol: ACIU
Event Phase: Suspended
Drug: Crenezumab
Indication: Alzheimer's Disease (AD)
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Amyloid Beta/Amyloid Plaques
Source Link: GlobeNewswire
[Continued for AC Immune SA.]
Clinical trials (LOA=likelihood of approval)
Corbus Pharmaceuticals Holdings, Inc.
Symbol: CRBP
Event Phase: IND
Drug: CRB-701
Indication: Solid Tumors
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Nectin-4
Drug Features: Antibody-Drug Conjugate
LOA: CSPC Pharmaceutical Group Limited (1093)
Source Link: GlobeNewswire
Johnson & Johnson
Symbol: JNJ
Event Phase: Approved
Drug: Erleada
Indication: Prostate Cancer
Lead Indication: Y
Molecule: Small Molecule
Target: Androgen receptors
LOA: 100%
Partner Companies: Nippon Shinyaku Co., Ltd. (4516), Royalty Pharma plc (RPRX)
Source Link: ASCO
Faron Pharmaceuticals Oy
Symbol: FARN
Event Phase: II
Drug: Bexmarilimab
Indication: Myelodysplastic Syndrome (MDS)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Clever-1/Stabilin-1/Feel-1, Immune System
Drug Features: Immuno-Oncology
LOA: 11%
Source Link: GlobeNewswire
Harpoon Therapeutics
Symbol: HARP
Event Phase: II
Drug: HPN328
Indication: Small Cell Lung Cancer (SCLC)
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Delta-like 3 (DLL3), Human Serum Albumin (HSA), T lymphocytes, Tumor Cells
Drug Features: Immuno-Oncology, Trispecific
LOA: 11%
Source Link: ASCO
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: I
Drug: BMS-986365
Indication: Prostate Cancer
Lead Indication: Y
Molecule: Small Molecule
Target: Androgen receptors
LOA: 5%
Source Link: ASCO
[Continued for Bristol Myers Squibb Company.]
Vera Therapeutics, Inc.
Symbol: VERA
Event Phase: III
Drug: Atacicept
Indication: Immunoglobulin A (IgA) Nephropathy (Berger's Disease)
Lead Indication: N
Molecule: Protein
Target: APRIL, B-cell activating factor (BAFF)/B-lymphocyte stimulator (BLyS)
LOA: 45%
Partner Companies: Bristol Myers Squibb Company (BMY), Merck KGaA (MKKGY)
Source Link: GlobeNewswire
Cytokinetics, Inc.
Symbol: CYTK
Event Phase: III
Drug: Aficamten
Indication: Cardiomyopathy - Hypertrophic
Lead Indication: Y
Molecule: Small Molecule
Target: Myosin
LOA: 54%
Partner Companies: Ji Xing Pharmaceuticals, Royalty Pharma plc (RPRX)
Source Link: GlobeNewswire
Bayer AG
Symbol: BAYN
Event Phase: Approved
Drug: Xofigo
Indication: Prostate Cancer
Lead Indication: Y
Molecule: Small Molecule
Target: DNA, Tumor Cells
Drug Features: Radiopharmaceutical
LOA: 100%
Source Link: ASCO
[Continued for Bayer AG.]
Merck & Co., Inc.
Symbol: MRK
Event Phase: III
Drug: MK-5684
Indication: Prostate Cancer
Lead Indication: Y
Molecule: Small Molecule
Target: Cytochrome p450
LOA: 44%
Partner Companies: Orion Corporation (ORINY)
Source Link: GlobeNewswire
Moleculin Biotech, Inc.
Symbol: MBRX
Event Phase: II
Drug: Annamycin
Indication: Acute Myelogenous Leukemia (AML)
Lead Indication: Y
Molecule: Small Molecule with Liposomal Delivery System
Target: Topoisomerase II (DNA gyrase)
LOA: 11%
Partner Companies: University of Texas MD Anderson Cancer Center
Source Link: PR Newswire
[Continued for Moleculin Biotech, Inc.]
Diamyd Medical AB
Symbol: DMYDB
Event Phase: III
Drug: Diamyd
Indication: Diabetes Mellitus, Type I
Lead Indication: Y
Molecule: Vaccine
Target: Glutamic Acid Decarboxylase (GAD), Immune System
LOA: 47%
Source Link: Cision
Awakn Life Sciences Corp.
Symbol: AWKN
Event Phase: Development Outside U.S.
Drug: AWKN-002
Indication: Alcohol Use Disorder
Lead Indication: N
Molecule: Small Molecule
Target: NMDA Glutamate Receptor
Drug Features: Reformulation
LOA: LTS Lohmann Therapie-Systeme AG
Source Link: BioSpace
Silo Pharma, Inc.
Symbol: SILO
Event Phase: Preclinical
Drug: SPC-14
Indication: Alzheimer's Disease (AD)
Lead Indication: N
Molecule: Small Molecule
Target: Serotonin 5-HT4 receptor
Drug Features: 505b2
Source Link: GlobeNewswire
Eli Lilly and Company
Symbol: LLY
Event Phase: II
Drug: AK-OTOF
Indication: Otoferlin Gene-Mediated Hearing Loss
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Otoferlin
LOA: 24%
Source Link: Lilly Investor
Ensysce Biosciences, Inc.
Symbol: ENSC
Event Phase: II
Drug: PF614
Indication: Moderate to Severe Pain
Lead Indication: Y
Molecule: Small Molecule
Target: Opioid receptors
Drug Features: 505b2, Reformulation
LOA: 12%
Source Link: AccessWire
Financing events
ArriVent Biopharma (AVBP):
Description: Clinical-stage biopharmaceutical company focusing on differentiated medicines for cancer.
Verticals: Life Sciences, Oncology
Deal Date: 26-janv-2024
Deal Type: IPO
Deal Size: $175.00 million
Investors: Not specified
Deal Synopsis: Raised $175 million in IPO on Nasdaq, under ticker symbol AVBP, with 9,722,222 shares sold at $18 per share.
Adicet Bio (ACET):
Description: Clinical-stage biotechnology company developing allogeneic gamma delta T cell therapies.
Verticals: Life Sciences, Oncology
Deal Date: 25-janv-2024
Deal Type: Public Investment 2nd Offering
Deal Size: $64.93 million
Investors: Not specified
Deal Synopsis: Raised $64.93 million in a second public offering on Nasdaq under the ticker symbol ACET, with 27,054,667 shares sold at $2.40 per share.
Adolore Biotherapeutics:
Description: Biotechnology company developing therapies for chronic pain and nervous system conditions.
Verticals: Life Sciences, LOHAS & Wellness
Deal Date: 25-janv-2024
Deal Type: Early Stage VC
Deal Size: $1.06 million
Investors: Undisclosed
Deal Synopsis: Raised $1.06 million in venture funding through convertible debt.
CG Oncology (CGON):
Description: Clinical-stage biopharmaceutical agency developing oncolytic immunotherapy.
Verticals: Life Sciences, Oncology
Deal Date: 25-janv-2024
Deal Type: IPO
Deal Size: $380.00 million
Investors: Not specified
Deal Synopsis: Raised $380 million in IPO on NASDAQ under the ticker symbol CGON, with 20,000,000 shares sold at $19 per share.
PurCell Bio:
Description: Operator of a cell culture research company providing chemically-defined cell culture media.
Verticals: Life Sciences
Deal Date: 25-janv-2024
Deal Type: Later Stage VC
Deal Size: $0.70 million
Investors: Undisclosed
Deal Synopsis: Raised $695,000 in venture funding from undisclosed investors.
Scout Bio:
Description: Developer of vector-delivered protein therapeutics for veterinary medicine.
Verticals: Life Sciences
Deal Date: 25-janv-2024
Deal Type: Buyout/LBO
Deal Size: Not specified
Investors: AQUITI Gestion, Ceva Sante Animale, Continental Grain Company, and others
Deal Synopsis: Acquired by Ceva Sante Animale through an LBO for an undisclosed amount.
Elo Life Systems:
Description: Precision gene editing company focusing on sustainable healthy food.
Verticals: AgTech, FoodTech, Life Sciences
Deal Date: 24-janv-2024
Deal Type: Early Stage VC
Deal Size: $20.50 million
Investors: Accelr8, Alexandria Venture Investments, DCVC Bio, and others
Deal Synopsis: Raised $20.5 million in Series A2 venture funding.
Kura Oncology (KURA):
Description: Clinical-stage biopharmaceutical company developing therapeutics for solid tumors and blood cancers.
Verticals: Life Sciences, Oncology
Deal Date: 24-janv-2024
Deal Type: PIPE
Deal Size: $150.00 million
Investors: EcoR1 Capital, Suvretta Capital Management
Deal Synopsis: In talks to receive $150 million of development capital through a private placement.
Neucore Bio:
Description: Developer of engineered delivery technologies for precision therapeutic innovation.
Verticals: Life Sciences
Deal Date: 24-janv-2024
Deal Type: Early Stage VC
Deal Size: Not specified
Investors: Rev1 Ventures
Deal Synopsis: Raised an undisclosed amount of venture funding from Rev1 Ventures.
Synnovation Therapeutics:
Description: Developer of precision therapies targeting highly validated disease targets.
Verticals: Life Sciences, Oncology
Deal Date: 24-janv-2024
Deal Type: Early Stage VC
Deal Size: $102.00 million
Investors: Cormorant Asset Management, Lilly Asia Ventures, NexTech, and others
Deal Synopsis: Raised $102 million in Series A venture funding.
Reduction in force (RIF)
January 22 - Cara Therapeutics: The pruritus-focused drug developer is laying off up to half of its staff as it narrows its clinical ambitions. The restructuring will extend Cara's cash runway into 2026. Story
Disease of the week
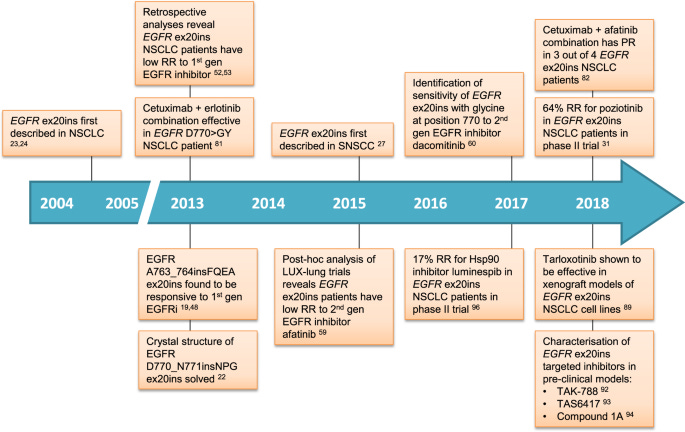
Exon 20 mutations in non-small cell lung cancer (NSCLC) are specific genetic alterations that occur in the 20th exon of certain genes. NSCLC is the most common type of lung cancer, and understanding the genetic mutations that drive its development is crucial for personalized treatment strategies.
Genes and Mutation Types:
EGFR Gene: Exon 20 mutations often involve the epidermal growth factor receptor (EGFR) gene, a tyrosine kinase receptor that regulates cell growth.
Other Genes: Besides EGFR, exon 20 mutations can also occur in other genes, such as HER2 (human epidermal growth factor receptor 2) and MET.
Less Common but Unique:
Exon 20 mutations are less common than mutations in other regions of the EGFR gene, such as exons 19 and 21. This makes them somewhat unique and distinct in terms of their clinical implications.
Treatment Challenges:
Historically, NSCLC patients with EGFR mutations have been treated with EGFR tyrosine kinase inhibitors (TKIs) like gefitinib, erlotinib, and afatinib. However, exon 20 mutations have shown resistance to these conventional EGFR TKIs.
The resistance of exon 20 mutations to standard EGFR TKIs poses a challenge in the treatment of NSCLC patients with this specific mutation.
Emerging Therapies:
The unique challenges posed by exon 20 mutations have led to increased research and development of targeted therapies specifically designed for this mutation.
Novel EGFR TKIs, designed to target exon 20 mutations, are being developed and tested in clinical trials. Drugs like mobocertinib and amivantamab-vmjw have shown promise in early studies.
Clinical Trials:
Numerous clinical trials are ongoing to evaluate the efficacy of various targeted therapies in treating NSCLC with exon 20 mutations.
Results from these trials are providing valuable insights into the effectiveness of different treatment approaches and helping shape future treatment strategies.
Prognosis and Patient Management:
The prognosis for NSCLC patients with exon 20 mutations may differ from those with other EGFR mutations.
Patient management involves a multidisciplinary approach, with oncologists considering the specific mutation, stage of cancer, and individual patient factors when determining the most appropriate treatment plan.
Ongoing Research:
Research in the field of oncology is dynamic, and ongoing efforts are being made to deepen our understanding of exon 20 mutations in NSCLC.
As more knowledge is gained, new therapeutic options and strategies may emerge to improve outcomes for patients with this specific genetic alteration.
What I’ve read this week
*Click on the pic to read*