Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Large Catalysts
Reminder
Some October-December Catalysts to Prepare in Advance
Conference for the Rest of the Year
PDUFA Dates
Most Shorted Biotech Stock as of This Week
Market
Notes : FDMT,KRYS,RCKT,BBIO,CABA
Venture Capital Market
M&A
How the market performed this week
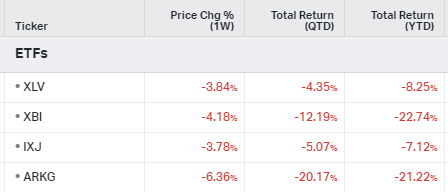
ETFs
Gene Therapy
RNA Therapy
Big Pharma
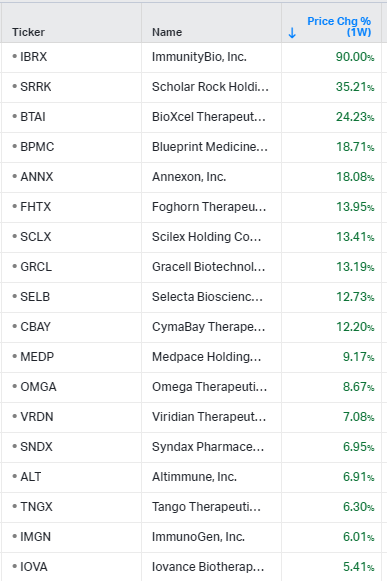
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Bristol Myers Squibb Company (BMY)
Event Phase: NDA/BLA
Drug: Camzyos
Indication: Cardiomyopathy - Hypertrophic
Lead Indication: Y
Molecule: Small Molecule
Target: Myosin
LOA : 82%
Partner Companies: LianBio (LIAN)
Source Link: LianBio Enters into Agreement with Bristol Myers Squibb for Mavacamten in China and Other Asian Markets
Bristol Myers Squibb Company (BMY)
Event Phase: NDA/BLA
Drug: Camzyos
Indication: Chronic Heart Failure - Preserved Ejection Fraction (Chronic HFpEF)
Lead Indication: N
Molecule: Small Molecule
Target: Myosin
LOA : 82%
Partner Companies: LianBio (LIAN)
Source Link: LianBio Enters into Agreement with Bristol Myers Squibb for Mavacamten in China and Other Asian Markets
Pfizer Inc. (PFE)
Event Phase: II
Drug: RVT-3101
Indication: Crohn's Disease
Lead Indication: Y
Molecule: Not Specified
Target: TNF superfamily member 15 (TNFSF15)
LOA : 19%
Partner Companies: Roivant Sciences Ltd. (ROIV), Telavant Holdings, Inc.
Cybin Inc. (CYBN)
Event Phase: Development Outside U.S.
Drug: SPL026
Indication: Major Depressive Disorder (MDD)
Lead Indication: Y
Molecule: Small Molecule
Target: Unknown
Source Link: Cybin Reports Positive Phase 2 Study Results of SPL026 in Major Depressive Disorder
Cybin Inc. (CYBN)
Event Phase: Development Outside U.S.
Drug: SPL028
Indication: Major Depressive Disorder (MDD)
Lead Indication: N
Molecule: Small Molecule
Target: Unknown
Source Link: Cybin Reports Positive Phase 2 Study Results of SPL028 in Major Depressive Disorder
Quince Therapeutics, Inc. (QNCX)
Event Phase: III
Drug: EryDex
Indication: Ataxia Telangiectasia
Lead Indication: Y
Molecule: Small Molecule
Target: Glucocorticoid Receptor (GR)
LOA : 52%
Source Link: Quince Therapeutics Reports Positive Phase 3 Results of EryDex in Ataxia Telangiectasia
Quince Therapeutics, Inc. (QNCX)
Event Phase: I
Drug: EryDex
Indication: Duchenne Muscular Dystrophy (DMD)
Lead Indication: N
Molecule: Small Molecule
Target: Glucocorticoid Receptor (GR)
LOA : 15%
Source Link: Quince Therapeutics Initiates Phase 1 Study of EryDex in Duchenne Muscular Dystrophy
Quince Therapeutics, Inc. (QNCX)
Event Phase: II
Drug: EE-TP
Indication: Metabolic - General
Lead Indication: N
Molecule: Small Molecule
Target: Unknown
LOA : 25%
Partner Companies: Orphan Technologies Ltd.
Source Link: Quince Therapeutics Initiates Phase 2 Study of EE-TP in Metabolic - General
Quince Therapeutics, Inc. (QNCX)
Event Phase: Preclinical
Drug: Ery-PAL
Indication: Phenylketonuria (PKU)
Lead Indication: N
Molecule: Small Molecule
Target: Unknown
Source Link: Quince Therapeutics Advances Preclinical Development of Ery-PAL for Phenylketonuria
Quince Therapeutics, Inc. (QNCX)
Event Phase: Preclinical
Drug: Ery-uricase
Indication: Gout
Lead Indication: N
Molecule: Small Molecule
Target: Unknown
Source Link: Quince Therapeutics Advances Preclinical Development of Ery-uricase for Gout
Quince Therapeutics, Inc. (QNCX)
Event Phase: Preclinical
Drug: Ery-GAMT
Indication: Metabolic - General
Lead Indication: N
Molecule: Small Molecule
Target: Unknown
Source Link: Quince Therapeutics Advances Preclinical Development of Ery-GAMT for Metabolic - General
Quince Therapeutics, Inc. (QNCX)
Event Phase: Preclinical
Drug: Ery-CoCe
Indication: Substance Use Disorder
Lead Indication: N
Molecule: Small Molecule
Target: Unknown
Source Link: Quince Therapeutics Advances Preclinical Development of Ery-CoCe for Substance Use Disorder
Clinical trials (LOA=likelihood of approval)
INmune Bio, Inc. (INMB)
Event Phase: II
Drug: XPro-1595
Indication: Alzheimer's Disease (AD)
Lead Indication: Y
Molecule: Protein
Target: Tumor Necrosis Factor-alpha (TNF-alpha)
LOA : 12%
Partner Companies: FPRT Bio, Xencor, Inc. (XNCR)
Acumen Pharmaceuticals, Inc. (ABOS)
Event Phase: I
Drug: ACU-193
Indication: Alzheimer's Disease (AD)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Amyloid Beta/Amyloid Plaques
LOA : 6%
Alnylam Pharmaceuticals Inc. (ALNY)
Event Phase: Development Outside U.S.
Drug: Onpattro
Indication: Transthyretin Amyloid Cardiomyopathy (ATTR-CM, Wild Type Or Hereditary)
Lead Indication: N
Molecule: siRNA/RNAi
Target: Transthyretin (TTR)
Partner Companies: Arbutus Biopharma Corporation (ABUS), GENESIS Pharma S.A., Ionis Pharmaceuticals, Inc. (IONS), Medison Pharma Ltd., Sanofi (SNY), taiba-ME
Source Link: Alnylam Pharmaceuticals Inc.
Bristol Myers Squibb Company (BMY)
Event Phase: Suspended
Drug: Sotyktu
Indication: Ulcerative Colitis (UC)
Lead Indication: N
Molecule: Small Molecule
Target: JAK/STAT, Tyrosine kinase 2 (TYK2)
Source Link: Bristol Myers Squibb Company
NKGen Biotech, Inc. (NKGNW)
Event Phase: I
Drug: SNK01
Indication: Alzheimer's Disease (AD)
Lead Indication: N
Molecule: Cellular
Target: Immune System, Natural Killer Cells (NK Cells)
LOA : 6%
BioNTech SE (BNTX)
Event Phase: II
Drug: BNT162b2+BNT161
Indication: Influenza (including vaccines)
Lead Indication: N
Molecule: mRNA (messenger RNA)
Target: Immune System, Influenza Virus, SARS-CoV-2
LOA : 23%
Partner Companies: Pfizer Inc. (PFE)
Source Link: BioNTech SE
Sernova Corp (SVA)
Event Phase: II
Drug: Sernova Cell Pouch System
Indication: Diabetes Mellitus, Type I
Lead Indication: Y
Molecule: Cellular
Target: Insulin Receptor
LOA : 15%
Partner Companies: Evotec SE (EVT)
Santhera Pharmaceuticals (SANN)
Event Phase: Approved
Drug: Agamree
Indication: Duchenne Muscular Dystrophy (DMD)
Lead Indication: Y
Molecule: Steroid
Target: Glucocorticoid Receptor (GR)
LOA : 100%
Partner Companies: Catalyst Pharmaceuticals, Inc. (CPRX), Idorsia Pharmaceuticals Ltd (IDIA), Johnson & Johnson (JNJ), Reveragen BioPharma, Inc., Sperogenix Therapeutics Limited
Source Link: Santhera Receives U.S. FDA Approval of AGAMREE (vamorolone) for the Treatment of Duchenne Muscular Dystrophy
Regeneron Pharmaceuticals, Inc. (REGN)
Event Phase: II
Drug: DB-OTO
Indication: Otoferlin Gene-Mediated Hearing Loss
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Otoferlin
LOA : 24%
Source Link: Regeneron Shares Preliminary Results Showing Gene Therapy
ESSA Pharma Inc. (EPIX)
Event Phase: II
Drug: Masofaniten
Indication: Prostate Cancer
Lead Indication: Y
Molecule: Small Molecule
Target: Androgen receptors
LOA : 11%
TransCode Therapeutics Inc (RNAZ)
Event Phase: I
Drug: TTX-MC138
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Lead Indication: N
Molecule: Small Molecule
Target: microRNA (miRNA)
LOA : 5%
Tonix Pharmaceuticals Holding Corp. (TNXP)
Event Phase: II
Drug: TNX-1900
Indication: Migraine and Other Headaches
Lead Indication: Y
Molecule: Small Molecule
Target: Oxytocin Receptor
LOA : 12%
Orchard Therapeutics Limited (ORTX)
Event Phase: II
Drug: OTL-203
Indication: Mucopolysaccharidosis I (MPS I; Hurler Syndrome)
Lead Indication: Y
Molecule: Non-Viral Gene Therapy
LOA : 25%
Partner Companies: Fondazione Telethon
Eli Lilly and Company (LLY)
Event Phase: Approved
Drug: Mirikizumab
Indication: Ulcerative Colitis (UC)
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: IL-23 (Interleukin-23)
LOA (Level of Advancement): 105%
GSK plc (GSK)
Event Phase: Approved
Drug: Arexvy
Indication: Respiratory Syncytial Virus (RSV) Prevention
Lead Indication: Y
Molecule: Vaccine
Target: Immune System, RSV, Respiratory Syncytial Virus
LOA : 100%
Partner Companies: Agenus Inc. (AGEN)
Source Link: Agenus Inc. Announces Positive Phase 3 Results for Balstilimab and Zalifrelimab in Cervical Cancer
Centessa Pharmaceuticals plc (CNTA)
Event Phase: Preclinical
Drug: ORX750
Indication: Narcolepsy
Lead Indication: Y
Molecule: Small Molecule
Target: Hypocretin/orexin receptor
Partner Companies: Sosei Heptares (4565)
Alnylam Pharmaceuticals Inc. (ALNY)
Event Phase: Development Outside U.S.
Drug: ALN-APP
Indication: Alzheimer's Disease (AD)
Lead Indication: Y
Molecule: siRNA/RNAi
Target: Amyloid Precursor Protein (APP)
Partner Companies: Regeneron Pharmaceuticals, Inc. (REGN)
Source Link: Alnylam Pharmaceuticals Inc.
BioVie, Inc. (BIVI)
Event Phase: III
Drug: NE3107
Indication: Alzheimer's Disease (AD)
Lead Indication: Y
Molecule: Small Molecule
Target: Tumor Necrosis Factor-alpha (TNF-alpha)
LOA : 46%
Freeline Therapeutics Holdings PLC (FRLN)
Event Phase: II
Drug: FLT201
Indication: Gaucher's Disease
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Recombinant adeno-associated virus (rAAV)
LOA : 27%
Skye Bioscience, Inc. (SKYE)
Event Phase: IND
Drug: SBI-100
Indication: Glaucoma / Ocular Hypertension (Ophthalmology)
Lead Indication: Y
Molecule: Small Molecule
Target: Cannabinoid-1 (CB1) receptor, Cannabinoid-2 (CB2) receptor
Partner Companies: Nanomerics, Ltd.
AlzeCure Pharma AB (ALZCUR)
Event Phase: Development Outside U.S.
Drug: ACD-856
Indication: Alzheimer's Disease (AD)
Lead Indication: N
Molecule: Small Molecule
Target: Trk (Tropomyosin Receptor Kinase) Receptors
Source Link: AlzeCure Pharma AB
Beam Therapeutics Inc. (BEAM)
Event Phase: Preclinical
Drug: BEAM-301
Indication: Glycogen Storage Disease (GSD)
Lead Indication: Y
Molecule: Non-Viral Gene Therapy
Target: Glucose 6-phosphatase
Biogen, Inc. (BIIB)
Event Phase: II
Drug: BIIB080
Indication: Alzheimer's Disease (AD)
Lead Indication: N
Molecule: Antisense
Target: RNA, Tau proteins
LOA : 13%
Partner Companies: Ionis Pharmaceuticals, Inc. (IONS)
CervoMed, Inc. (CRVO)
Event Phase: II
Drug: Neflamapimod
Indication: Lewy Body Dementia (LBD)
Lead Indication: N
Molecule: Small Molecule
Target: p38 MAP kinase (MAPK)
LOA : 15%
Partner Companies: Vertex Pharmaceuticals Incorporated (VRTX)
Cassava Sciences, Inc. (SAVA)
Event Phase: III
Drug: Simufilam
Indication: Alzheimer's Disease (AD)
Lead Indication: N
Molecule: Small Molecule
Target: Filamin A (FLNA)
LOA : 41%
Source Link: MRI Data Suggest Simufilam is Not Associated with Amyloid-related Imaging Abnormalities (ARIA)
AbbVie Inc. (ABBV)
Event Phase: III
Drug: AGN-151586
Indication: Wrinkles
Lead Indication: Y
Molecule: Natural Protein
Target: Botulinum toxin
LOA : 67%
Partner Companies: Bonti, Inc.
Avadel Pharmaceuticals plc (AVDL)
Event Phase: Approved
Drug: Lumryz
Indication: Narcolepsy
Lead Indication: Y
Molecule: Small Molecule
Target: GABA Receptors, GABA-B Receptor
LOA : 100%
Bristol Myers Squibb Company (BMY)
Event Phase: III
Drug: BMS-986278
Indication: Idiopathic Pulmonary Fibrosis (IPF)
Lead Indication: Y
Molecule: Small Molecule
Target: Lysophosphatidic acid receptor 1 (LPAR1)
LOA : 62%
Source Link: Bristol Myers Squibb Company
TransCode Therapeutics Inc (RNAZ)
Event Phase: IND
Drug: TTX-MC138
Indication: Solid Tumors
Lead Indication: N
Molecule: Small Molecule
Target: microRNA (miRNA)
Quoin Pharmaceuticals, Ltd. (QNRX)
Event Phase: III
Drug: QRX003
Indication: Congenital Ichthyosis
Lead Indication: Y
Molecule: Not Specified
Partner Companies: AFT Pharmaceuticals Limited (AFT), Endo International plc (ENDP), Er-Kim Ilac, Genpharm Services, Neopharm Medical Supplies & Commerce Ltd., OrphanDC, Orpharm LLC, Winhealth Pharma Group
Idorsia Pharmaceuticals Ltd (IDIA)
Event Phase: Approved
Drug: Quviviq
Indication: Insomnia
Lead Indication: Y
Molecule: Small Molecule
Target: Hypocretin/orexin receptor
LOA : 100%
Partner Companies: Mochida Pharmaceutical Co., Ltd. (4534), Simcere Pharmaceutical Group (2096), Sosei Heptares (4565)
Source Link: Idorsia Pharmaceuticals Ltd
Axsome Therapeutics, Inc. (AXSM)
Event Phase: III
Drug: AUVELITY
Indication: Neuropsychiatric Symptoms in Alzheimer's Disease
Lead Indication: N
Molecule: Small Molecule
Target: Dopamine Reuptake, Nicotinic Acetylcholine Receptors (nAChR), NMDA Glutamate Receptor, Norepinephrine (Noradrenaline) Reuptake/Transporter, Serotonin Receptors - Unspecified, Sigma-1 Receptor
LOA : 59%
Landos Biopharma, Inc. (LABP)
Event Phase: II
Drug: NX-13
Indication: Ulcerative Colitis (UC)
Lead Indication: N
Molecule: Small Molecule
Target: Mitochondria
LOA : 19%
Partner Companies: LianBio (LIAN)
Source Link: Landos Biopharma Presents Clinical Trial Data for Ulcerative Colitis Candidate NX-13
Roche Holding AG (RHHBY)
Event Phase: Approved
Drug: Tecentriq
Indication: Small Cell Lung Cancer (SCLC)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Immune System, Programmed death-ligand 1 (PD-L1)
LOA : 100%
Partner Companies: Bristol Myers Squibb Company (BMY), Chugai Pharmaceutical Co., Ltd. (4519), Ono Pharmaceutical Company, Ltd. (4528:JP)
Source Link: Roche's Tecentriq Approved for Small Cell Lung Cancer
KalVista Pharmaceuticals, Inc. (KALV)
Event Phase: III
Drug: Sebetralstat
Indication: Hereditary Angioedema (HAE)
Lead Indication: Y
Molecule: Small Molecule
Target: Kinin-Kallikrein System
LOA : 66%
Source Link: KalVista Pharmaceuticals Announces Positive Phase 3 Results for Sebetralstat in Hereditary Angioedema (HAE)
Cognition Therapeutics, Inc. (CGTX)
Event Phase: II
Drug: Elayta
Indication: Alzheimer's Disease (AD)
Lead Indication: Y
Molecule: Small Molecule
Target: Amyloid Beta/Amyloid Plaques
LOA : 13%
MeiraGTx Holdings plc (MGTX)
Event Phase: II
Drug: AAV-AQP1
Indication: Xerostomia (Dry Mouth)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Aquaporins
LOA : 20%
Partner Companies: National Institute of Dental and Craniofacial Research
Johnson & Johnson (JNJ)
Event Phase: I
Drug: NBTXR3 (Drug)
Indication: Pancreatic Cancer
Lead Indication: N
Molecule: Small Molecule
Target: Immune System, Tumor Cells
LOA : 5%
Partner Companies: LianBio (LIAN), Nanobiotix SA (NBTX)
IO Biotech Inc. (IOBT)
Event Phase: II
Drug: IO102-IO103
Indication: Solid Tumors
Lead Indication: N
Molecule: Peptide
Target: IDO (Indoleamine 2,3-dioxygenase), Immune System, Programmed death-ligand 1 (PD-L1)
LOA : 11%
BerGenBio ASA (BGBIO)
Event Phase: II
Drug: Bemcentinib
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: N
Molecule: Small Molecule
Target: Axl Receptor Tyrosine Kinase
LOA: 14%
Partner Companies: Rigel Pharmaceuticals, Inc. (RIGL)
Source Link: New Data on BerGenBio's Selective Axl Inhibitor Bemcentinib Released at 2023 ESMO Meeting
BioNTech SE (BNTX)
Event Phase: II
Drug: DB-1305
Indication: Solid Tumors
Lead Indication: Y
Molecule: Small Molecule
Target: Unknown
LOA : 11%
Partner Companies: Duality Biologics
Source Link: BioNTech SE
Amgen, Inc. (AMGN)
Event Phase: III
Drug: Bemarituzumab
Indication: Gastric Cancer
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Fibroblast Growth Factor Receptor-2
LOA : 49%
Partner Companies: Zai Lab Ltd. (ZLAB)
Source Link: Amgen's Bemarituzumab for Gastric Cancer
Apollomics, Inc. (APLM)
Event Phase: Preclinical
Drug: APL-101
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: N
Molecule: Small Molecule
Target: Hepatocyte growth factor receptor (c-Met, HGFR)
Partner Companies: Hengkang Medical Group Co Ltd (002219), Zhejiang Bossan Pharmaceutical Co., Ltd
IDEAYA Biosciences, Inc. (IDYA)
Event Phase: II
Drug: IDE196
Indication: Uveal Melanoma
Lead Indication: Y
Molecule: Small Molecule
Target: Protein Kinase C (PKC)
LOA : 13%
Partner Companies: Novartis AG (NVS), Roche Holding AG (RHHBY)
AstraZeneca PLC (AZN)
Status: Suspended
Drug: Monalizumab
Indication: Head and Neck Cancer
New Drug: N
Drug Type: Monoclonal Antibody
Target: Immune System, Natural Killer Cells (NK Cells), Natural Killer Group 2A (NKG2A)
Collaborators: Innate Pharma S.A. (IPHA), Novo Nordisk A/S (NVO)
Link: Monalizumab Presentation
Avadel Pharmaceuticals plc (AVDL)
Status: Approved
Drug: Lumryz
Indication: Narcolepsy
New Drug: Y
Drug Type: Small Molecule
Target: GABA Receptors, GABA-B Receptor
Collaboration: 100%
Link: Lumryz Presentation
Eli Lilly and Company (LLY)
Status: Approved
Drug: Verzenio
Indication: HR+/HER2- Breast Cancer
New Drug: Y
Drug Type: Small Molecule
Target: Cyclin Dependent Kinase 4 (CDK-4), Cyclin Dependent Kinase 6 (CDK-6)
Collaboration: 100%
Link: Verzenio Presentation
Johnson & Johnson (JNJ)
Status: Approved
Drug: Rybrevant
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: Y
Drug Type: Monoclonal Antibody
Target: EGFR (Epidermal Growth Factor Receptor), Hepatocyte growth factor receptor (c-Met, HGFR)
Collaboration: 100%
Collaborator: Genmab A/S (GMAB)
Link: Rybrevant Presentation
BioNTech SE (BNTX)
Status: II
Drug: BNT211
Indication: Solid Tumors
New Drug: Y
Drug Type: Cellular
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), CLDN6, Immune System, T lymphocytes
Collaboration: 11%
Link: BNT211 Presentation
Johnson & Johnson (JNJ)
Status: Approved
Drug: Rybrevant
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: Y
Drug Type: Monoclonal Antibody
Target: EGFR (Epidermal Growth Factor Receptor), Hepatocyte growth factor receptor (c-Met, HGFR)
Collaboration: 100%
Collaborator: Genmab A/S (GMAB)
Link: Rybrevant Presentation
Merck KGaA (MKKGY)
Status: Approved
Drug: Tepmetko
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: Y
Drug Type: Small Molecule
Target: Hepatocyte growth factor receptor (c-Met, HGFR)
Collaboration: 100%
Link: Tepmetko Presentation
Avadel Pharmaceuticals plc (AVDL)
Status: Approved
Drug: Lumryz
Indication: Narcolepsy
New Drug: Y
Drug Type: Small Molecule
Target: GABA Receptors, GABA-B Receptor
Collaboration: 100%
Link: Lumryz Presentation
Merck & Co., Inc. (MRK)
Status: Approved
Drug: Keytruda
Indication: Esophageal Cancer
New Drug: N
Drug Type: Monoclonal Antibody
Target: Immune System, Programmed death-1 receptor (PD-1)
Collaboration: 100%
Collaborator: DRI Capital Inc.
Link: Keytruda Presentation
Amgen, Inc. (AMGN)
Status: Development Outside U.S.
Drug: Xgeva
Indication: Renal Cell Cancer (RCC)
New Drug: N
Drug Type: Monoclonal Antibody
Target: RANK Ligand (RANKL)
Collaboration: AstraZeneca PLC (AZN), BeiGene, Ltd. (BGNE), Daiichi Sankyo Co., Ltd. (4568), Dr. Reddy's Laboratories Ltd. (RDY)
Link: Xgeva Presentation
Coherus BioSciences, Inc. (CHRS)
Status: II
Drug: Toripalimab
Indication: Solid Tumors
New Drug: N
Drug Type: Monoclonal Antibody
Target: Immune System, Programmed death-1 receptor (PD-1)
Collaboration: 11%
Collaborators: AstraZeneca PLC (AZN), Excellmab Pte. Ltd., Hikma Pharmaceuticals plc (HIK), Shanghai Junshi Biosciences Co., Ltd. (1877)
Link: Toripalimab Presentation
BioNTech SE (BNTX)
Status: Preclinical
Drug: BNT314
Indication: Solid Tumors
New Drug: N
Drug Type: Monoclonal Antibody
Target: Unknown
Link: BNT314 Presentation
Bolt Biotherapeutics, Inc. (BOLT)
Status: II
Drug: BDC-1001
Indication: Solid Tumors
New Drug: Y
Drug Type: Monoclonal Antibody
Target: HER2/neu or ErbB-2, Immune System, Toll-like receptor 7 (TLR7), Toll-like receptor 8 (TLR8)
Collaboration: 11%
Link: BDC-1001 Presentation
Ayala Pharmaceuticals, Inc. (ADXS)
Status: III
Drug: AL102
Indication: Desmoid Tumors
New Drug: N
Drug Type: Small Molecule
Target: Gamma-secretase, Notch Receptors
Collaboration: 45%
CymaBay Therapeutics, Inc. (CBAY)
Status: III
Drug: Seladelpar
Indication: Primary Biliary Cholangitis (PBC)
New Drug: N
Drug Type: Small Molecule
Target: PPAR delta
Collaboration: 81%
Link: Seladelpar Granted Revised Breakthrough Therapy Designation
Novartis AG (NVS)
Status: Approved
Drug: Pluvicto
Indication: Prostate Cancer
New Drug: Y
Drug Type: Small Molecule
Target: Prostate-specific Membrane Antigen (PSMA)
Collaboration: 100%
Link: Novartis Pluvicto Shows Clinically Meaningful and Highly Statistically Significant RPFS Benefit
Exscientia plc (EXAI)
Status: Preclinical
Drug: EXS-73565
Indication: Hematologic Cancer
New Drug: N
Drug Type: Small Molecule
Target: MALT1
Link: EXS-73565 Presentation
AstraZeneca PLC (AZN)
Status: Approved
Drug: Imfinzi
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: N
Drug Type: Monoclonal Antibody
Target: Immune System, Programmed death-ligand 1 (PD-L1)
Collaboration: 100%
Collaborators: Bristol Myers Squibb Company (BMY), Eli Lilly and Company (LLY)
Link: Imfinzi Presentation
MAIA Biotechnology, Inc. (MAIA)
Status: II
Drug: THIO
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: Y
Drug Type: Small Molecule
Target: DNA synthesis, Telomeres
Collaboration: 11%
Link: THIO Presentation
BioNTech SE (BNTX)
Status: II
Drug: BNT211
Indication: Testicular Cancer
New Drug: N
Drug Type: Cellular
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), CLDN6, Immune System, T lymphocytes
Collaboration: 11%
Link: BNT211 Presentation
BioNTech SE (BNTX)
Status: I
Drug: BNT-221
Indication: Melanoma
New Drug: Y
Drug Type: Cellular
Target: Stem Cells/Other Cell Therapies, T-Cell Receptor (TCR)
Collaboration: 5%
Link: BNT-221 Presentation
AstraZeneca PLC (AZN)
Status: Approved
Drug: Imfinzi
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: N
Drug Type: Monoclonal Antibody
Target: Immune System, Programmed death-ligand 1 (PD-L1)
Collaboration: 100%
Collaborators: Bristol Myers Squibb Company (BMY), Eli Lilly and Company (LLY)
Link: Imfinzi Presentation
Merck & Co., Inc. (MRK)
Status: Approved
Drug: Keytruda
Indication: Melanoma
New Drug: Y
Drug Type: Monoclonal Antibody
Target: Immune System, Programmed death-1 receptor (PD-1)
Collaboration: 100%
Collaborator: DRI Capital Inc.
Link: Keytruda Presentation
Novartis AG (NVS)
Status: Approved
Drug: Tabrecta
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: Y
Drug Type: Small Molecule
Target: Hepatocyte growth factor receptor (c-Met, HGFR)
Collaboration: 100%
Collaborator: Incyte Corporation (INCY)
Link: Tabrecta Presentation
BridgeBio Pharma, Inc. (BBIO)
Status: Development Outside U.S.
Drug: Truseltiq
Indication: Gastric Cancer
New Drug: N
Drug Type: Small Molecule
Target: Fibroblast Growth Factor Receptor (FGFR)
Collaborators: Helsinn Healthcare SA, Juniper Biologics Pte Ltd, LianBio (LIAN), Novartis AG (NVS)
Link: LianBio Announces Presentation of Data from Phase 2a Study of Infigratinib
Adaptimmune Therapeutics plc (ADAP)
Status: I
Drug: ADP-A2M4CD8
Indication: Solid Tumors
New Drug: N
Drug Type: Cellular
Target: Cluster of Differentiation 107a (CD107a) / LAMP-1, Cluster of Differentiation 8 (CD8), Melanoma antigen-encoding gene (MAGE), Stem Cells/Other Cell Therapies, T-Cell Receptor (TCR)
Collaboration: 5%
Alkermes plc (ALKS)
Status: Development Outside U.S.
Drug: ALKS 2680
Indication: Narcolepsy
New Drug: Y
Drug Type: Small Molecule
Target: Hypocretin/orexin receptor
Link: Alkermes Presents First Clinical Data for Orexin-2 Receptor Agonist ALKS 2680
Johnson & Johnson (JNJ)
Status: III
Drug: Tremfya
Indication: Ulcerative Colitis (UC)
New Drug: N
Drug Type: Monoclonal Antibody
Target: IL-23 (Interleukin-23)
Collaboration: 72%
Collaborators: MorphoSys AG (MOR), Otsuka Holdings Co., Ltd. (4578)
Theriva Biologics, Inc. (TOVX)
Status: I
Drug: VCN-01
Indication: Head and Neck Cancer
New Drug: N
Drug Type: Viral
Target: Hyaluronic acid, Oncolytic Virus Therapy
Collaboration: 5%
Collaborator: Grifols, S.A. (GRFS)
Link: Theriva Biologics Presents Survival Outcomes Data from Phase 1 Study
Ryvu Therapeutics (RVU)
Status: Development Outside U.S.
Drug: SEL120
Indication: Solid Tumors
New Drug: N
Drug Type: Small Molecule
Target: Cyclin Dependent Kinase 8 (CDK-8), Immune System
Link: Ryvu Therapeutics Provides an Update on the Progress of RVU120
Oncolytics Biotech, Inc. (ONC)
Status: III
Drug: Pelareorep
Indication: Pancreatic Cancer
New Drug: N
Drug Type: Viral
Target: Immune System, Oncolytic Virus Therapy, Ras
Collaboration: 43%
Collaborator: Adlai Nortye Biopharma Co., Ltd. (ANL)
Link: Oncolytics Presents Positive Updated Pancreatic Cancer Data from Goblet Phase 1/2 Study at ESMO
Merck & Co., Inc. (MRK)
Status: II
Drug: DS-7300
Indication: Solid Tumors
New Drug: Y
Drug Type: Monoclonal Antibody
Target: B7-H3
Collaboration: 12%
Collaborator: Daiichi Sankyo Co., Ltd. (4568)
Link: DS-7300 Presentation
Amgen, Inc. (AMGN)
Status: Approved
Drug: Vectibix
Indication: Colorectal Cancer (CRC)
New Drug: Y
Drug Type: Monoclonal Antibody
Target: EGFR (Epidermal Growth Factor Receptor)
Collaboration: 100%
Collaborators: Beta Pharma, Inc., Takeda Pharmaceutical Co. Ltd. (TAK)
Link: Vectibix Presentation
Merck & Co., Inc. (MRK)
Status: Approved
Drug: Keytruda
Indication: Solid Tumors
New Drug: N
Drug Type: Monoclonal Antibody
Target: Immune System, Programmed death-1 receptor (PD-1)
Collaboration: 100%
Collaborator: DRI Capital Inc.
AstraZeneca PLC (AZN)
Status: Approved
Drug: Imfinzi
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: N
Drug Type: Monoclonal Antibody
Target: Immune System, Programmed death-ligand 1 (PD-L1)
Collaboration: 100%
Collaborators: Bristol Myers Squibb Company (BMY), Eli Lilly and Company (LLY)
Link: DUART Presentation
Galecto Inc. (GLTO)
Status: Development Outside U.S.
Drug: GB1211
Indication: Non-Small Cell Lung Cancer (NSCLC)
New Drug: N
Drug Type: Small Molecule
Target: Galectin-3
Link: GB1211 Presentation
Merck & Co., Inc. (MRK)
Status: I
Drug: MK-1084
Indication: Solid Tumors
New Drug: Y
Target: Not Specified
Collaboration: 5%
Link: MK-1084 Presentation
Ascendis Pharma A/S (ASND)
Status: II
Drug: TransCon IL-2
Indication: Solid Tumors
New Drug: Y
Drug Type: Small Molecule
Target: IL-2 (Interleukin-2)
Collaboration: 11%
Link: Ascendis Presents Updated and New TransCon IL-2 β γ Monotherapy and Combination Therapy Data
Financing events
IDEAYA Biosciences (NAS: IDYA)
Description: Oncology-focused precision medicine company
Verticals: Life Sciences, Oncology
Deal Date: 26-oct-2023
Deal Type: Public Investment 2nd Offering
Deal Size: $117.50 million
Investors: Not specified
Deal Synopsis: Raised $117.5 million in a second public offering on Nasdaq.
Loop Dee Science
Description: Developer of molecular biology diagnostics kits
Verticals: Life Sciences
Deal Date: 26-oct-2023
Deal Type: Later Stage VC
Deal Size: EUR 1.90 million
Investors: Crédit Agricole Aquitaine, GO Capital (France), Normandie Business Angels, Normandie Participations
Deal Synopsis: Raised EUR 1.8 million in venture funding for molecular biology diagnostics kits.
Paion (ETR: PA8)
Description: Medical innovations for procedural sedation, anesthesia, and critical care services
Verticals: Life Sciences
Deal Date: 26-oct-2023
Deal Type: Bankruptcy: Liquidation
Deal Size: Not specified
Investors: Not specified
Deal Synopsis: In talks to get liquidated as of October 26, 2023.
RAYDIAX
Description: Developer of a therapeutic imaging system for minimally invasive tumor interventions
Verticals: Life Sciences, Oncology
Deal Date: 26-oct-2023
Deal Type: Seed Round
Deal Size: EUR 2.54 million
Investors: bmp Ventures (Philipp Kopp), High-Tech Gründerfonds, IBG Beteiligungsgesellschaft Sachsen-Anhalt
Deal Synopsis: Raised EUR 2.4 million in seed funding for a therapeutic imaging system.
Triveni Bio
Description: Developer of a drug discovery platform for I&I disorders
Verticals: Life Sciences
Deal Date: 26-oct-2023
Deal Type: Early Stage VC
Deal Size: $92.00 million
Investors: Alexandria Venture Investments, Atlas Venture, Cormorant Asset Management, OrbiMed, Polaris Partners, The Invus Group, Viking Global Investors
Deal Synopsis: Raised $92 million in Series A venture funding for drug discovery platform.
Amneal Pharmaceuticals (NYS: AMRX)
Description: Generic pharmaceutical manufacturer
Verticals: Life Sciences, Manufacturing
Deal Date: 25-oct-2023
Deal Type: Debt Refinancing
Deal Size: Not specified
Investors: Not specified
Deal Synopsis: Entered into a debt refinancing round on October 25, 2023.
ImmuONE
Description: Provider of in vitro cell culture services for safety assessment
Verticals: Life Sciences
Deal Date: 25-oct-2023
Deal Type: Later Stage VC
Deal Size: GBP 2.44 million
Investors: Mercia Asset Management (LON: MERC), Midlands Engine Investment Fund, Pioneer Group (London)
Deal Synopsis: Raised GBP 2 million in venture funding for in vitro cell culture services.
Triplebar
Description: Biotechnology firm focusing on food sustainability
Verticals: CleanTech, HealthTech, Life Sciences
Deal Date: 25-oct-2023
Deal Type: Early Stage VC
Deal Size: $19.76 million
Investors: Not specified
Deal Synopsis: Raised $19.76 million in Series A venture funding for food sustainability.
Adial Pharmaceuticals (NAS: ADIL)
Description: Clinical-stage biopharmaceutical company for addiction and related disorders
Verticals: Life Sciences
Deal Date: 24-oct-2023
Deal Type: PIPE
Deal Size: $4.00 million
Investors: Not specified
Deal Synopsis: Received $4 million of development capital through a private placement.
Aiolos Bio
Description: Clinical-stage biopharmaceutical company for respiratory and inflammatory conditions
Verticals: Life Sciences
Deal Date: 24-oct-2023
Deal Type: Early Stage VC
Deal Size: $245.00 million
Investors: Atlas Venture, Bain Capital Life Sciences, Forbion, RA Capital Management, Sofinnova Investments
Deal Synopsis: Raised $245 million in Series A venture funding.
DermBiont
Description: Developer of microbial and targeted topical therapeutics for skin diseases
Verticals: Life Sciences
Deal Date: 24-oct-2023
Deal Type: Later Stage VC
Deal Size: $35.20 million
Investors: Civilization Ventures, Double Point Ventures (Daniel Yadegar), Olive Tree Capital, Viking Global Investors
Deal Synopsis: Raised $35.2 million in Series B venture funding.
Infirst Healthcare
Description: Producer of medicines for common cough, inflammatory pain, and chronic wounds
Verticals: Life Sciences
Deal Date: 24-oct-2023
Deal Type: Buyout/LBO
Deal Size: Not specified
Investors: Carlin Consumer Health (Scott Chapman)
Deal Synopsis: Acquired by Carlin Consumer Health through an LBO.
LyGenesis
Description: Developer of cell therapy technology for organ regeneration and transplant
Verticals: Life Sciences
Deal Date: 24-oct-2023
Deal Type: Later Stage VC
Deal Size: $19.00 million
Investors: Juvenescence (Richard Marshall), Prime Movers Lab (Justin Briggs)
Deal Synopsis: Raised $19 million in Series A2 venture funding.
Nykode (OSL: NYKD)
Description: Clinical-stage biopharmaceutical company for immunotherapies
Verticals: Life Sciences, Oncology
Deal Date: 24-oct-2023
Deal Type: PIPE
Deal Size: NOK 46.01 million
Investors: Not specified
Deal Synopsis: Received NOK 505 million of development capital through a private placement.
Palatin Technologies (ASE: PTN)
Description: Developer of receptor-specific peptide therapeutics
Verticals: Life Sciences
Deal Date: 24-oct-2023
Deal Type: PIPE
Deal Size: $2.12 million
Investors: Not specified
Deal Synopsis: Received $2.12 million of development capital through a private placement.
Switchback Systems
Description: Developer of DNA synthesis platform
Verticals: Life Sciences
Deal Date: 24-oct-2023
Deal Type: Early Stage VC
Deal Size: $6.72 million
Investors: OMX Ventures (Craig Asher), Vertical Venture Partners (Paul Conley), Ahren Innovation Capital
Deal Synopsis: Raised $6.72 million in venture funding.
Aligos Therapeutics (NAS: ALGS)
Description: Clinical-stage biopharmaceutical company for viral and liver diseases
Verticals: Life Sciences, Oncology
Deal Date: 23-oct-2023
Deal Type: PIPE
Deal Size: $92.00 million
Investors: Not specified
Deal Synopsis: In talks to receive $92 million of development capital through a private placement.
Arcutis Biotherapeutics (NAS: ARQT)
Description: Medical dermatology company
Verticals: Life Sciences
Deal Date: 23-oct-2023
Deal Type: Public Investment 2nd Offering
Deal Size: $81.25 million
Investors: Not specified
Deal Synopsis: Raised $81.25 million in its second public offering on Nasdaq.
Califia Pharma
Description: Developer of drugs for TCR-deficient solid tumors
Verticals: Life Sciences, Oncology
Deal Date: 23-oct-2023
Deal Type: Seed Round
Deal Size: $1.20 million
Investors: Ulu Ventures, SeedFolio
Deal Synopsis: Raised $1.20 million in seed funding.
ENSEM Therapeutics
Description: Developer of precision drugs for oncology therapies
Verticals: Artificial Intelligence & Machine Learning, Life Sciences, Oncology
Deal Date: 23-oct-2023
Deal Type: Early Stage VC
Deal Size: $10.00 million
Investors: Not specified
Deal Synopsis: Raised $10 million in Series A+ venture funding.
Harpoon Therapeutics (NAS: HARP)
Description: Clinical-stage immunotherapy company
Verticals: Life Sciences, Oncology
Deal Date: 23-oct-2023
Deal Type: PIPE
Deal Size: $150.00 million
Investors: The Invus Group, Ally Bridge Group, Soleus Capital, K2 HealthVentures, RA Capital Management, Commodore Capital, New Leaf Venture, Cormorant Asset Management, Lion Point Capital, Surveyor Capital
Deal Synopsis: In talks to receive $150 million of development capital through a private placement.
NAYA Biosciences
Description: Biotechnology company for oncology, fertility, and regenerative medicine
Verticals: Artificial Intelligence & Machine Learning, Life Sciences, Oncology
Deal Date: 23-oct-2023
Deal Type: Reverse Merger
Deal Size: Not specified
Investors: Invo Bioscience (NAS: INVO) (Steven Shum)
Deal Synopsis: Reached a definitive agreement to acquire Invo Bioscience through a reverse merger.
Small Pharma
Description: Neuropharmaceutical company for mental health disorders
Verticals: Life Sciences
Deal Date: 23-oct-2023
Deal Type: Merger/Acquisition
Deal Size: Not specified
Investors: Cybin (NEOE: CYBN) (Douglas Drysdale)
Deal Synopsis: Acquired by Cybin (NEOE: CYBN) for an undisclosed amount.
SPARTA Biodiscovery
Description: Provider of nanoparticle analytical testing services
Verticals: Life Sciences, Nanotechnology
Deal Date: 23-oct-2023
Deal Type: Corporate
Deal Size: GBP 3.5 million
Investors: Sartorius (ETR: SRT) (Oscar-Werner Reif)
Deal Synopsis: A minority stake acquired by Sartorius for GBP 3.5 million.
Telavant
Description: Developer of innovative drugs and therapies for inflammatory and fibrotic diseases
Verticals: HealthTech, Life Sciences
Deal Date: 23-oct-2023
Deal Type: Merger/Acquisition
Deal Size: $7.25 billion
Investors: Roche (SWX: ROG)
Deal Synopsis: Reached a definitive agreement to be acquired by Roche for $7.25 billion.
Reduction in force (RIF)
October 26 - ElevateBio: Even with $401 million in the bank—thanks to one of the year’s largest biotech fundraisings—ElevateBio is shaving off some pre-clinical work resulting in layoffs that will affect 13% of the workforce. It’s unclear exactly how many employees will be headed out the door. The company is listed as having around 500 people on LinkedIn. Story
October 24 - Amgen: After a $27.8 billion buyout of Horizon, Amgen is laying off 350 former Horizon employees. The positions that are being eliminated are jobs that overlap with existing teams at Amgen. Story
October 24 - Idorsia: The insomnia-focused biotech is laying off 300 people, predominantly in R&D and associated functions. About 175 other positions were made redundant by either canceling open positions or not replacing people who had left. The workforce reduction follows Idorsia's warning in July that it may have to let go of up to 500 people to shave costs. Story
October 24 - BrainStorm Cell Therapeutics: After failing to win approval for its stem cell therapy candidate, NurOwn, in amyotrophic lateral sclerosis, BrainStorm is laying off 30% of its staff, including Chief Medical Officer Kirk Taylor, M.D. The move is part of a broader effort to eliminate activities outside of what it will need to try to overcome the FDA’s reservations regarding existing evidence on NurOwn. Story
Disease of the week
Leprosy, also known as Hansen's disease, is a chronic infectious disease caused by the bacterium Mycobacterium leprae. It primarily affects the skin, nerves, and mucous membranes and can lead to significant disfigurement and disability if left untreated. Leprosy is one of the oldest known diseases in human history, and although it is curable, it has been associated with social stigma and discrimination throughout history.
Niche reference :
Here's a comprehensive overview of leprosy, covering its causes, symptoms, transmission, diagnosis, treatment, and historical and social aspects:
Causes: Leprosy is caused by the slow-growing bacterium Mycobacterium leprae. The exact mode of transmission is not fully understood, but it is believed to occur through prolonged close contact with an infected person, as the bacterium is not highly contagious. Leprosy primarily affects the skin and nerves.
Symptoms: Leprosy can manifest in various forms, but it is typically classified into two main types:
Tuberculoid Leprosy: Characterized by limited skin lesions and nerve involvement, which can lead to sensory loss in affected areas.
Lepromatous Leprosy: This form has widespread skin lesions, nodules, and pronounced nerve damage. It is more severe than the tuberculoid form.
Common symptoms include skin lesions, numbness, and muscle weakness. Left untreated, it can lead to deformities, blindness, and other complications.
Transmission: Leprosy is thought to spread through respiratory droplets from an infected person, but it requires prolonged and close contact for transmission. It is not as highly contagious as some other infectious diseases.
Diagnosis: Diagnosis is typically made through clinical evaluation, skin biopsies, and the examination of skin smears for the presence of M. leprae. The disease is usually categorized into paucibacillary (few bacteria) and multibacillary (many bacteria) forms based on the number of skin lesions and bacteria present.
Treatment: Leprosy is curable with multidrug therapy (MDT), which typically includes a combination of antibiotics like dapsone, rifampicin, and clofazimine. The treatment duration can be several months to a few years, depending on the severity of the disease.
Prevention: There is no known vaccine to prevent leprosy. Early detection and treatment of cases, along with contact tracing and chemoprophylaxis for those in close contact with infected individuals, are key strategies for preventing the spread of the disease.
Historical and Social Aspects: Leprosy has been associated with social stigma and discrimination for centuries. In many cultures, individuals with leprosy were historically isolated or ostracized, contributing to the perception of the disease as "unclean." Efforts to combat this stigma have been ongoing for many years.
Leprosy is mentioned in various historical and religious texts, and leper colonies were established in many countries to segregate those with the disease. However, advancements in treatment and a better understanding of the disease have led to the de-stigmatization of leprosy in many parts of the world.
Global Status: Leprosy remains a public health issue in some countries, with the highest burden in India, Brazil, and Indonesia. Efforts are ongoing to eliminate the disease as a public health problem, and many countries have made significant progress in reducing leprosy cases.
Research and Advocacy: Several organizations, including the World Health Organization (WHO) and the Novartis Foundation, have been working to raise awareness, provide treatment, and reduce the stigma associated with leprosy. Research into better diagnostic tools and treatments also continues.