Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
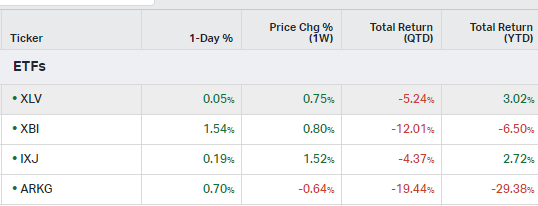
How the market performed this week
ETFs
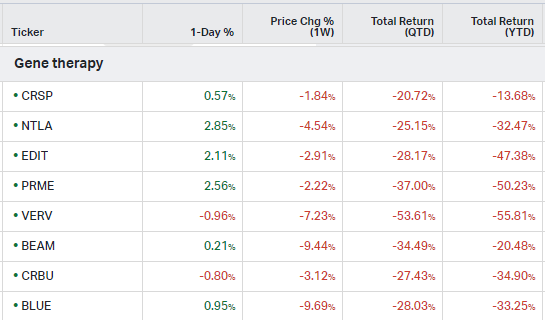
Gene Therapy
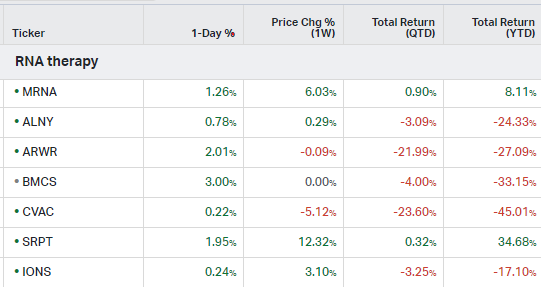
RNA Therapy
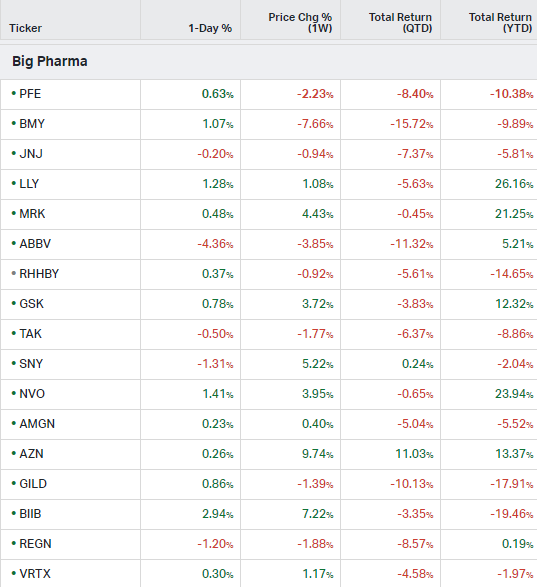
Big Pharma
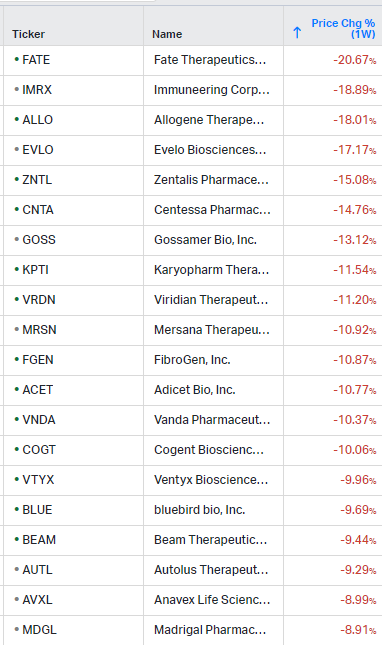
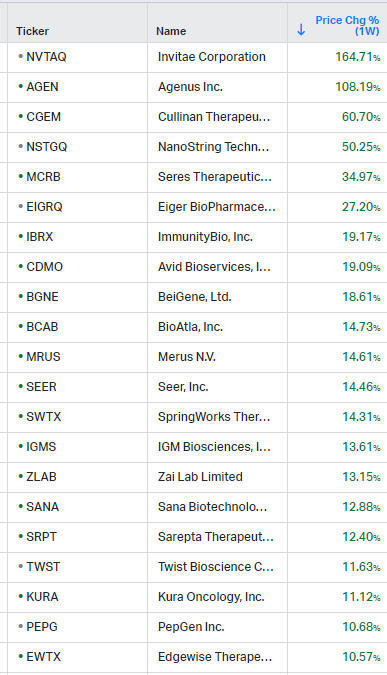
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Day One Biopharmaceuticals, LLC
Symbol: DAWN
Event Phase: Approved
Current Phase: Approved
Drug: Ojemda
Disease Group: Oncology
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Lead Indication: Yes
Rare Disease: Yes
Target: Raf kinase
LOA: 100%
Source Link: Link
Cidara Therapeutics, Inc.
Symbol: CDTX
Event Phase: II
Current Phase: I/II
Drug: CD388
Disease Group: Infectious Disease
Indication: Influenza (including vaccines)
Lead Indication: Yes
Rare Disease: N/A
Target: Immune System, Influenza Virus
LOA: 23%
Source Link: Link
Petros Pharmaceuticals, Inc.
Symbol: PTPI
Event Phase: Approved
Current Phase: Approved
Drug: Stendra
Disease Group: Urology
Indication: Erectile Dysfunction (ED)
Lead Indication: Yes
Rare Disease: N/A
Target: Phosphodiesterase 5 (PDE5)
LOA: 100%
Source Link: Link
Hanmi Pharmaceutical Co., Ltd.
Symbol: 128940
Event Phase: I
Current Phase: I
Drug: BH3120
Disease Group: Oncology
Indication: Solid Tumors
Lead Indication: No
Rare Disease: N/A
Target: CD137 (4-1BB ; TNFRS9), Immune System, Programmed death-ligand 1 (PD-L1)
LOA: 5%
Source Link: Link
Mallinckrodt plc
Symbol: MNKKQ
Event Phase: II
Current Phase: II
Drug: Xenex
Disease Group: Cardiovascular
Indication: Cardiac Arrest/Resuscitation
Lead Indication: Yes
Rare Disease: N/A
Target: NMDA Glutamate Receptor
LOA: 10%
Source Link: Link
Lexeo Therapeutics
Symbol: LXEO
Event Phase: II
Current Phase: I/II
Drug: LX2006
Disease Group: Neurology
Indication: Friedreich's Ataxia
Lead Indication: Yes
Rare Disease: Yes
Target: Frataxin (FXN)
LOA: 12%
Source Link: Link
NeuroSense Therapeutics Ltd.
Symbol: NRSN
Event Phase: Development Outside U.S.
Current Phase: Development Outside U.S.
Drug: PrimeC
Disease Group: Neurology
Indication: Alzheimer's Disease (AD)
Lead Indication: No
Rare Disease: N/A
Target: Cyclooxygenase 2 (COX2) / Prostaglandin-Endoperoxide Synthase 2 (PTGS2), Topoisomerase II (DNA gyrase) and IV
LOA: [Not Provided]
Source Link: Link
Clinical trials (LOA=likelihood of approval)
Gilead Sciences, Inc.
Symbol: GILD
Event Type: Regulatory - Change to Product Label (U.S.)
Event Phase: Approved
Drug: Biktarvy
Disease Group: Infectious Disease
Indication: HIV / AIDS Treatment
Target: HIV Integrase
LOA: 100%
Source Link: Link
Pfizer Inc.
Symbol: PFE
Event Type: Regulatory - Approval (U.S.)
Event Phase: Approved
Drug: Beqvez
Disease Group: Hematology
Indication: Hemophilia B
Target: Coagulation Factor IX
LOA: 100%
Source Link: Link
AbbVie Inc.
Symbol: ABBV
Event Type: Trial Data - Top-Line Results
Event Phase: Approved
Drug: Rinvoq
Disease Group: Allergy
Indication: Atopic Dermatitis (Eczema)
Target: JAK/STAT
LOA: 100%
Source Link: Link
ImmunityBio Inc.
Symbol: IBRX
Event Type: Trial Data - Updated Results
Event Phase: III
Drug: Anktiva
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: IL-15 (Interleukin-15)/IL-15 Receptor, Immune System
LOA: 44%
Source Link: Link
Eyenovia, Inc.
Symbol: EYEN
Event Type: Trial Data - Top-Line Results
Event Phase: Approved
Drug: MydCombi
Disease Group: Ophthalmology
Indication: Other Ophthalmological Indications (Ophthalmology)
Target: Alpha 1 Adrenergic Receptor, Muscarinic acetylcholine receptor
LOA: 100%
Source Link: Link
VistaGen Therapeutics, Inc.
Symbol: VTGN
Event Type: Trial Data - Top-Line Results
Event Phase: II
Drug: PH15
Disease Group: Neurology
Indication: Neurology - Other
Target: Unknown
LOA: 12%
Source Link: Link
Calliditas Therapeutics AB
Symbol: CALT
Event Type: Trial Data - Top-Line Results
Event Phase: Approved
Drug: Tarpeyo
Disease Group: Renal
Indication: Immunoglobulin A (IgA) Nephropathy (Berger's Disease)
Target: Glucocorticoid Receptor (GR), Immune System
LOA: 100%
Source Link: Link
Innoviva, Inc.
Symbol: INVA
Event Type: Trial Data - Updated Results
Event Phase: III
Drug: Zoliflodacin
Disease Group: Infectious Disease
Indication: Urinary Tract and Reproductive Tract Infections (Antibacterial)
Target: Gram-Negative Bacteria, Topoisomerase II (DNA gyrase)
LOA: 68%
Source Link: Link
Gain Therapeutics, Inc.
Symbol: GANX
Event Type: Trial Data - Top-Line Results
Event Phase: Development Outside U.S.
Drug: GT-02287
Disease Group: Neurology
Indication: Parkinson's Disease (PD)
Target: GBA2 (extralysosomal glucocerebrosidase)
LOA: [Not Provided]
Source Link: Link
Algernon Pharmaceuticals Inc.
Symbol: AGN
Event Type: Trial Data
Event Phase: Preclinical
Drug: AP-188
Disease Group: Cardiovascular
Indication: Ischemic Stroke
Target: Unknown
LOA: [Not Provided]
Source Link: Link
Kiromic BioPharma, Inc.
Symbol: KRBP
Event Type: Trial Data - Updated Results
Event Phase: I
Drug: Deltacel
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Unknown
LOA: 5%
Source Link: Link
Alvotech
Symbol: ALVO
Event Type: Trial Data - Top-Line Results
Event Phase: Development Outside U.S.
Drug: AVT05
Disease Group: Autoimmune/immunology
Indication: Rheumatoid Arthritis (RA)
Target: Tumor Necrosis Factor-alpha (TNF-alpha)
LOA: [Not Provided]
Source Link: Link
Spago Nanomedical AB.
Symbol: SPAG
Event Type: Trial Data - Preclinical Results
Event Phase: Development Outside U.S.
Drug: Tumorad
Disease Group: Oncology
Indication: Solid Tumors
Target: Unknown
LOA: [Not Provided]
Source Link: Link
Novartis AG
Symbol: NVS
Event Type: Trial Data - Top-Line Results
Event Phase: Approved
Drug: Coartem
Disease Group: Infectious Disease
Indication: Malaria
Target: Mitochondrial Electron Transport Chain, Reactive Oxygen Species/Free Radicals
LOA: 100%
Source Link: Link
BioVie, Inc.
Symbol: BIVI
Event Type: Trial Data - Updated Results
Event Phase: III
Drug: NE-3107
Disease Group: Neurology
Indication: Alzheimer's Disease (AD)
Target: Tumor Necrosis Factor-alpha (TNF-alpha)
LOA: 46%
Source Link: Link
Neurocrine Biosciences, Inc.
Symbol: NBIX
Event Type: Trial Data - Top-Line Results
Event Phase: II
Drug: NBI-1065845
Disease Group: Psychiatry
Indication: Major Depressive Disorder (MDD)
Target: AMPA-type glutamate receptor
LOA: 15%
Source Link: Link
Sanofi
Symbol: SNY
Event Type: Trial Data - Top-Line Results
Event Phase: III
Drug: PRN1008
Disease Group: Autoimmune/immunology
Indication: Immune Thrombocytopenic Purpura (ITP)
Target: Bruton's Tyrosine Kinase (BTK)
LOA: 61%
Source Link: Link
Alto Neuroscience
Symbol: ANRO
Event Type: Trial Data - Updated Results
Event Phase: I
Drug: ALTO-101
Disease Group: Psychiatry
Indication: Schizophrenia
Target: Phosphodiesterase 4 (PDE4)
LOA: 7%
Source Link: Link
Silo Pharma, Inc.
Symbol: SILO
Event Type: Trial Data - Preclinical Results
Event Phase: Preclinical
Drug: SPC-15
Disease Group: Psychiatry
Indication: Post-Traumatic Stress Disorder (PTSD)
Target: Serotonin 5-HT4 receptor
LOA: [Not Provided]
Source Link: Link
TME Pharma N.V.
Symbol: ALTME
Event Type: Trial Data - Updated Results
Event Phase: IND
Drug: NOX-A12
Disease Group: Oncology
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Target: Chemokine (C-X-C motif) Ligand 12 (CXCL12)/Stromal Cell-Derived Factor 1 (SDF-1)
LOA: [Not Provided]
Source Link: Link
Financing events
AccessHope
Description: Provides cancer breakthrough services, delivering expertise from a National Cancer Institute-designated comprehensive cancer center to cancer patients and their families.
Verticals: Life Sciences, Oncology
Deal Date: 22-Apr-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $33 million of Series B venture funding from City of Hope to accelerate innovation and expand into new markets.
Investors: City of Hope (Harlan Levine)
Deal Size: $33.00 million
Akava Therapeutics
Description: Develops small molecule therapeutics for neurodegenerative diseases and cancers, focusing on protein aggregation inhibition.
Verticals: Life Sciences, Oncology
Deal Date: 23-Apr-2024
Deal Type: Seed Round
Deal Synopsis: In process of raising $5 million of seed funding.
Investors: [Not specified]
Deal Size: $5.00 million
Anuncia Medical
Description: Develops a ventricular system for the treatment of hydrocephalus, focusing on managing cerebrospinal fluid and reducing complications from non-flowing shunts.
Verticals: HealthTech, Life Sciences
Deal Date: 24-Apr-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $3.41 million of venture funding through convertible debt from multiple investors.
Investors: Alliance of Angels, Ariel Savannah Angel Partners, Pimlico Pond Investments, Thornapple River Capital, Xcellerant Ventures
Deal Size: $3.41 million
AtaCor Medical
Description: Develops a medical device for cardiac pacing and rhythm management that eliminates the need for hardware inside or onto the heart.
Verticals: HealthTech, Life Sciences
Deal Date: 24-Apr-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $28 million of Series C venture funding to support FDA submission and a pilot study.
Investors: Arboretum Ventures (Jan Garfinkle), BayMed Venture Partners, Catalyst Health Ventures (Darshana Zaveri), Hatteras Venture Partners (Jeff Terrell), Long View Equity Partners
Deal Size: $28.00 million
BioMérieux
Description: Develops and manufactures in vitro diagnostics for detecting pathogens and contamination across multiple segments.
Verticals: Life Sciences, Manufacturing, Oncology
Deal Date: 22-Apr-2024
Deal Type: PIPE
Deal Synopsis: Dassault Group acquired a 0.8% stake in BioMérieux through a private placement.
Investors: Dassault Group
Deal Size: $104.16 million
Cerevance
Description: Develops novel therapeutics for central nervous system diseases, utilizing technology to differentiate specific cell types in mature human brains.
Verticals: Life Sciences
Deal Date: 25-Apr-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $163 million of Series B and B1 funding to advance programs focused on Parkinson's disease, ALS, and schizophrenia.
Investors: Agent Capital (Campbell Murray), Bioluminescence Ventures (Kouki Harasaki), Casdin Capital, Dolby Family Ventures, Double Point Ventures, Foresite Capital (James Tananbaum), Gates Frontier, GV, LifeRock Ventures, Lightstone Ventures (Jason Lettmann), MQB Partners, SV Health Investors (Laurence Barker), Takeda Ventures, UPMC Enterprises (Matthias Kleinz), William Gates (William Gates)
Deal Size: $163.00 million
Cidara Therapeutics
Description: Biotechnology company focused on the development and commercialization of novel anti-infectives.
Verticals: Life Sciences
Deal Date: 24-Apr-2024
Deal Type: PIPE
Deal Synopsis: Received $240 million from a private placement to fund an upfront payment under an agreement with Johnson & Johnson and support a Phase 2b trial.
Investors: Bain Capital Life Sciences, BVF Partners, Canaan Partners, RA Capital Management
Deal Size: $240.00 million
Reduction in force (RIF)
April 23 - Bristol-Myers Squibb: The big pharma plans to cut costs by $1.5 billion by the end of 2025 in a massive restructure that includes laying off about 2,200 employees. Story
April 23 - Tessera Therapeutics: About 13-14% of employees, or 50 people are being let go from Tessera as the biotech shifts from preclinical to clinical development. "As a result of positive data from our preclinical programs, we have reached an inflection point where we need to rebalance the resources of our organization to prioritize and grow our clinical development efforts in anticipation of advancing multiple candidates into the clinic," the company said in an April 24 statement.
April 23 - BenevolentAI: Eleven months after restructuring, the AI-enabled drug developer is pulling another pivot, axing plans to launch software products, laying off another 30% of staff and closing its U.S. office. Story
Disease of the week
Machado-Joseph Disease (MJD), also known as Spinocerebellar Ataxia Type 3 (SCA3), is a rare, inherited neurodegenerative disorder. It is one of the types of spinocerebellar ataxia and is characterized by the gradual impairment of muscular coordination (ataxia) and other aspects of nervous system function. MJD has an autosomal dominant pattern of inheritance, meaning that having just one copy of the mutated gene from one parent is sufficient to develop the disorder.
Genetic Background
MJD is caused by a mutation in the ATXN3 gene, located on chromosome 14. This gene mutation involves the expansion of a CAG trinucleotide repeat. In healthy individuals, the ATXN3 gene contains 12 to 44 CAG repeats, but in those with MJD, the repeat number can be significantly higher (typically 52 to 86 repeats). This leads to the production of an abnormally long version of the ataxin-3 protein, which accumulates in the brain and disrupts normal function.
Symptoms
Symptoms of MJD can vary widely among affected individuals, even within the same family. Common symptoms include:
Ataxia: Impairment of muscle coordination that can affect speech, eye movements, and limb movements.
Parkinsonism: Features resembling Parkinson’s disease such as rigidity and bradykinesia (slowness of movement).
Peripheral neuropathy: Abnormalities in nerve function outside the brain and spinal cord, leading to issues like altered sensation and weakness in the limbs.
Dystonia: Sustained muscle contractions causing twisting and repetitive movements or abnormal postures.
Ophthalmoplegia: Weakness or paralysis of the eye muscles.
Progression
MJD typically has an adult onset, with symptoms commonly appearing between the ages of 30 and 40, but onset can range from childhood to late adulthood. The disease progresses gradually over time, and the severity of symptoms can increase, leading to more pronounced disability. Life expectancy varies, and while MJD can shorten lifespan, many individuals live for several decades after the onset of symptoms.
Diagnosis
Diagnosis of MJD is primarily based on clinical examination and family history, given its genetic basis. Genetic testing can confirm the diagnosis by identifying the expanded CAG repeat in the ATXN3 gene.
Treatment and Management
Currently, there is no cure for MJD, and treatment focuses on managing symptoms and improving quality of life. Treatment may include:
Physical therapy to help maintain mobility and balance.
Speech therapy to manage difficulties with speech and swallowing.
Occupational therapy to assist with daily activities and recommend modifications to the living environment.
Medications may be used to manage specific symptoms such as spasticity, parkinsonism, or depression.
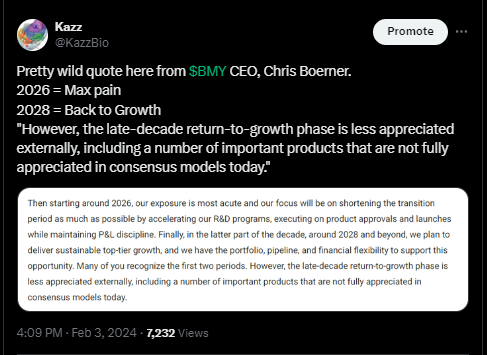
What I’ve read this week
*Click on the pic to read*