Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
How the market performed this week
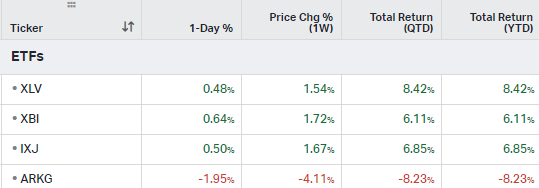
ETFs
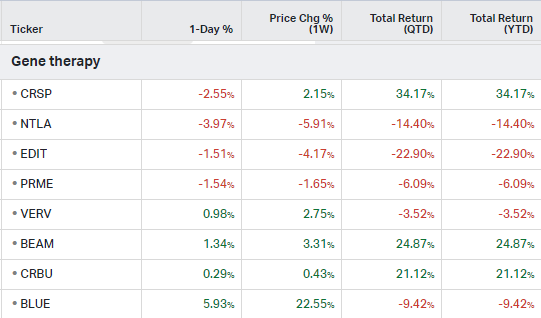
Gene Therapy
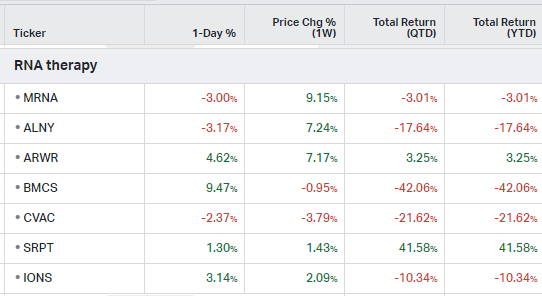
RNA Therapy
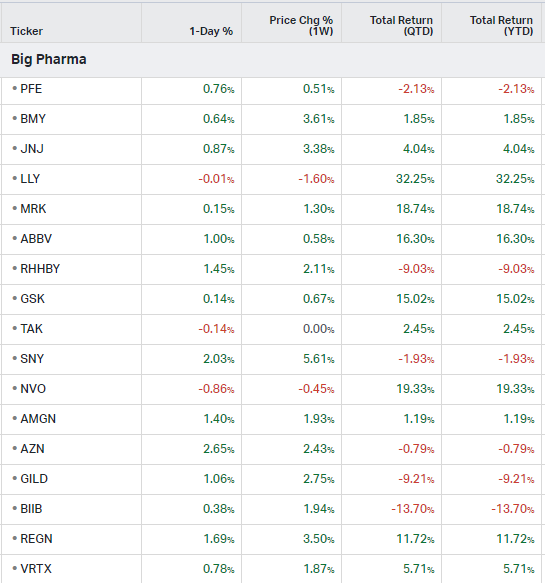
Big Pharma
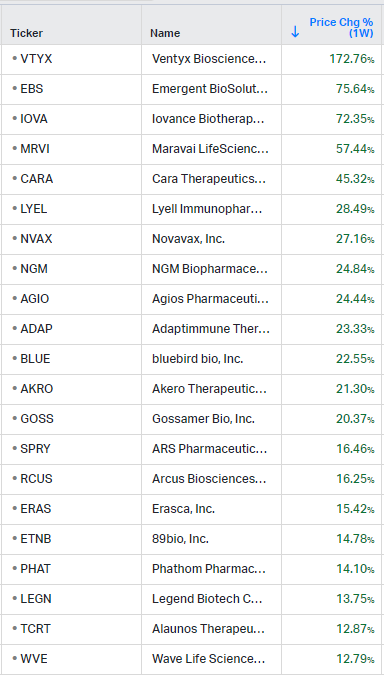
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
AstraZeneca PLC (AZN)
Lead Company: AstraZeneca PLC
Symbol: AZN
Event Phase: II
Drug: GC012F
Indication: Multiple Myeloma (MM)
Lead Indication: Y
Molecule: Cellular
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), B-cell maturation antigen (BCMA), Cluster of Differentiation 19 (CD19), Immune System
Drug Features: Immuno-Oncology
LOA: 12%
Partner Companies: Not specified
Source Link: Link
AstraZeneca PLC (AZN)
Lead Company: AstraZeneca PLC
Symbol: AZN
Event Phase: Development Outside U.S.
Drug: GC012F
Indication: Diffuse Large B-Cell Lymphoma (DLBCL) - NHL
Lead Indication: N
Molecule: Cellular
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), B-cell maturation antigen (BCMA), Cluster of Differentiation 19 (CD19), Immune System
Drug Features: Immuno-Oncology
LOA: Not specified
Partner Companies: Not specified
Source Link: Link
AstraZeneca PLC (AZN)
Lead Company: AstraZeneca PLC
Symbol: AZN
Event Phase: IND
Drug: GC012F
Indication: Systemic Lupus Erythematosus (SLE)
Lead Indication: N
Molecule: Cellular
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), B-cell maturation antigen (BCMA), Cluster of Differentiation 19 (CD19), Immune System
Drug Features: Immuno-Oncology
LOA: Not specified
Partner Companies: Not specified
Source Link: Link
AstraZeneca PLC (AZN)
Lead Company: AstraZeneca PLC
Symbol: AZN
Event Phase: Development Outside U.S.
Drug: GC007G
Indication: Acute Lymphoblastic Leukemia (ALL)
Lead Indication: Y
Molecule: Cellular
Target: Allogeneic Chimeric Antigen Receptor T-cells (CAR-T), Cluster of Differentiation 19 (CD19), Immune System
Drug Features: Immuno-Oncology
LOA: Not specified
Partner Companies: Not specified
Source Link: Link
AstraZeneca PLC (AZN)
Lead Company: AstraZeneca PLC
Symbol: AZN
Event Phase: Development Outside U.S.
Drug: GC-502
Indication: Acute Lymphoblastic Leukemia (ALL)
Lead Indication: Y
Molecule: Cellular
Target: Allogeneic Chimeric Antigen Receptor T-cells (CAR-T), Cluster of Differentiation 19 (CD19), Cluster of Differentiation 7 (CD7)
Drug Features: Immuno-Oncology
LOA: Not specified
Partner Companies: Not specified
Source Link: Link
AstraZeneca PLC (AZN)
Lead Company: AstraZeneca PLC
Symbol: AZN
Event Phase: Development Outside U.S.
Drug: GC-509
Indication: Acute Myelogenous Leukemia (AML)
Lead Indication: N
Molecule: Cellular
Target: Unknown
Drug Features: Immuno-Oncology
LOA: Not specified
Partner Companies: Not specified
Source Link: Link
AstraZeneca PLC (AZN)
Lead Company: AstraZeneca PLC
Symbol: AZN
Event Phase: Preclinical
Drug: GC-506
Indication: Solid Tumors
Lead Indication: N
Molecule: Cellular
Target: GC182 (CLDN18.2)
Drug Features: Immuno-Oncology
LOA: Not specified
Partner Companies: FutureGen Biopharm
Source Link: Link
Rigel Pharmaceuticals Acquires US Rights to Gavreto
Rigel Pharmaceuticals, Inc. (RIGL)
Lead Company: Rigel Pharmaceuticals, Inc.
Symbol: RIGL
Event Phase: Approved
Drug: Gavreto
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: Y
Molecule: Small Molecule
Target: RET
Drug Features: Not specified
LOA: 100%
Partner Companies: Blueprint Medicines Corporation (BPMC), CStone Pharmaceuticals (Suzhou) Co., Ltd (2616), Sinopharm Group Co. Ltd. (1099)
Source Link: Link
Rigel Pharmaceuticals, Inc. (RIGL)
Lead Company: Rigel Pharmaceuticals, Inc.
Symbol: RIGL
Event Phase: Approved
Drug: Gavreto
Indication: Thyroid Cancer
Lead Indication: N
Molecule: Small Molecule
Target: RET
Drug Features: Not specified
LOA: 100%
Partner Companies: Blueprint Medicines Corporation (BPMC), CStone Pharmaceuticals (Suzhou) Co., Ltd (2616), Sinopharm Group Co. Ltd. (1099)
Source Link: Link
Immunocore, Ltd. (IMCR)
Lead Company: Immunocore, Ltd.
Symbol: IMCR
Event Phase: II
Drug: IMC-F106C
Indication: Melanoma
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Immune System, T-Cell Receptor (TCR)
Drug Features: Bispecific, Immuno-Oncology
LOA: 11%
Partner Companies: Not specified
Source Link: Link
BioVaxys Technology Corp. (BIOV)
Lead Company: BioVaxys Technology Corp.
Symbol: BIOV
Event Phase: II
Drug: Maveropepimut-S
Indication: Diffuse Large B-Cell Lymphoma (DLBCL) - NHL
Lead Indication: Y
Molecule: Peptide
Target: Immune System, Survivin
Drug Features: Not specified
LOA: 13%
Partner Companies: Not specified
Source Link: Link
BioVaxys Technology Corp. (BIOV)
Lead Company: BioVaxys Technology Corp.
Symbol: BIOV
Event Phase: I
Drug: Maveropepimut-S
Indication: Bladder Cancer
Lead Indication: N
Molecule: Peptide
Target: Immune System, Survivin
Drug Features: Not specified
LOA: 5%
Partner Companies: Not specified
Source Link: Link
BioVaxys Technology Corp. (BIOV)
Lead Company: BioVaxys Technology Corp.
Symbol: BIOV
Event Phase: II
Drug: Maveropepimut-S
Indication: Ovarian Cancer
Lead Indication: N
Molecule: Peptide
Target: Immune System, Survivin
Drug Features: Not specified
LOA: 14%
Partner Companies: Not specified
Source Link: Link
BioVaxys Technology Corp. (BIOV)
Lead Company: BioVaxys Technology Corp.
Symbol: BIOV
Event Phase: I
Drug: DPX-SurMAGE
Indication: Bladder Cancer
Lead Indication: Y
Molecule: Peptide
Target: Melanoma antigen-encoding gene (MAGE), Survivin
Drug Features: Immuno-Oncology
LOA: 5%
Partner Companies: Not specified
Source Link: Link
Clinical trials (LOA=likelihood of approval)
SynAct Pharma AB
Symbol: SYNACT:SS
Event Phase: II
Drug: AP1189
Indication: Rheumatoid Arthritis (RA)
Lead Indication: N
Molecule: Small Molecule
Target: Melanocortin (MC) receptors
LOA: 19%
Partner Companies: None
Source Link: SynAct Pharma Announcement
Medivir AB
Symbol: MVIRB:SS
Event Phase: Preclinical
Drug: MIV-818
Indication: Hepatocellular (Liver) Cancer (HCC) (Including Secondary Metastases)
Lead Indication: Y
Molecule: Small Molecule
Target: DNA polymerase
LOA: Not specified
Partner Companies: None
Source Link: Medivir Announcement
Tarsus Pharmaceuticals Inc.
Symbol: TARS
Event Phase: II
Drug: TP-05
Indication: Antibacterial and Antifungal - Miscellaneous Vaccines and Treatments
Lead Indication: N
Molecule: Small Molecule
Target: GABA-A Receptor
LOA: 23%
Partner Companies: None
Source Link: Tarsus Pharmaceuticals Announcement
Abivax S.A.
Symbol: ABVX
Event Phase: III
Drug: Obefazimod
Indication: Ulcerative Colitis (UC)
Lead Indication: Y
Molecule: Small Molecule
Target: Cytoplasmic CAP Binding Complex (CBC80/20), microRNA (miRNA), miR-124
LOA: 67%
Partner Companies: None
Source Link: Abivax Announcement
AbbVie Inc.
Symbol: ABBV
Event Phase: NDA/BLA
Drug: Skyrizi
Indication: Ulcerative Colitis (UC)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: IL-23 (Interleukin-23)
LOA: 99%
Partner Companies: Boehringer Ingelheim GmbH
Source Link: AbbVie Announcement
Pharvaris B.V.
Symbol: PHVS
Event Phase: III
Drug: PHVS416
Indication: Hereditary Angioedema (HAE)
Lead Indication: Y
Molecule: Small Molecule
Target: Bradykinin B2 Receptor
LOA: 66%
Partner Companies: None
Source Link: Pharvaris Announcement
GSK plc
Symbol: GSK
Event Phase: II
Drug: Shigella vaccine (GSK)
Indication: Gastroenteritis
Lead Indication: Y
Molecule: Vaccine
Target: Immune System
LOA: 18%
Partner Companies: LimmaTech Biologics AG
Source Link: GSK Announcement
Avenue Therapeutics Inc.
Symbol: ATXI
Event Phase: Preclinical
Drug: BAER-101
Indication: Seizure Disorders (Epilepsy)
Lead Indication: N
Molecule: Small Molecule
Target: GABA Receptors
LOA: Not specified
Partner Companies: AstraZeneca PLC (AZN), Cincinnati Children's Hospital Medical Center
Source Link: Avenue Therapeutics Announcement
GSK plc
Symbol: GSK
Event Phase: Approved
Drug: Cabenuva
Indication: HIV / AIDS Treatment
Lead Indication: Y
Molecule: Small Molecule
Target: HIV Integrase
LOA: 100%
Partner Companies: Johnson & Johnson (JNJ)
Source Link: GSK Announcement
Chimerix, Inc.
Symbol: CMRX
Event Phase: II
Drug: Brincidofovir (IV)
Indication: Antiviral - Other Treatments
Lead Indication: N
Molecule: Small Molecule
Target: DNA polymerase
LOA: 23%
Partner Companies: SymBio Pharmaceuticals Limited (4582)
Source Link: Chimerix Announcement
CymaBay Therapeutics, Inc.
Symbol: CBAY
Event Phase: NDA/BLA
Drug: Seladelpar
Indication: Primary Biliary Cholangitis (PBC)
Lead Indication: N
Molecule: Small Molecule
Target: PPAR delta
LOA: 99%
Partner Companies: Johnson & Johnson (JNJ), Kaken Pharmaceutical Company, Ltd (4521)
Source Link: CymaBay Therapeutics Announcement
RAPT Therapeutics, Inc.
Symbol: RAPT
Event Phase: II
Drug: RPT193
Indication: Asthma
Lead Indication: N
Molecule: Small Molecule
Target: Chemokine Receptor 4 (CCR4)
LOA: 13%
Partner Companies: None
Source Link: RAPT Therapeutics Announcement
FibroBiologics, Inc.
Symbol: FBLG
Event Phase: I
Drug: CYMS-101
Indication: Multiple Sclerosis (MS)
Lead Indication: N
Molecule: Cellular
Target: Fibroblasts
LOA: 6%
Partner Companies: None
Source Link: FibroBiologics Announcement
Sanofi
Symbol: SNY
Event Phase: Suspended
Drug: TEV-48574
Indication: Asthma
Lead Indication: N
Molecule: Monoclonal Antibody
Target: TNF superfamily member 15 (TNFSF15)
LOA: Not specified
Partner Companies: Teva Pharmaceutical Industries Ltd. (TEVA)
Source Link: Sanofi Announcement
ARS Pharmaceuticals, Inc.
Symbol: SPRY
Event Phase: III
Drug: Neffy
Indication: Anaphylaxis
Lead Indication: Y
Molecule: Small Molecule
Target: Alpha Adrenergic Receptors, Beta Adrenergic Receptors
LOA: 65%
Partner Companies: None
Source Link: ARS Pharmaceuticals Announcement
Tharimmune, Inc.
Symbol: THAR
Event Phase: I
Drug: TH104
Indication: Pruritus
Lead Indication: N
Molecule: Small Molecule
Target: IL-17 (Interleukin 17), Opioid receptors
LOA: 10%
Partner Companies: Avior Inc.
Source Link: Tharimmune Announcement
Voyager Therapeutics, Inc
Symbol: VYGR
Event Phase: Preclinical
Drug: VY-TAU01
Indication: Alzheimer's Disease (AD)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Tau proteins
LOA: Not specified
Partner Companies: None
Source Link: Voyager Therapeutics Announcement
Johnson & Johnson
Symbol: JNJ
Event Phase: Approved
Drug: Carvykti
Indication: Multiple Myeloma (MM)
Lead Indication: Y
Molecule: Cellular
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), B-cell maturation antigen (BCMA), Immune System, Stem Cells/Other Cell Therapies
LOA: 100%
Partner Companies: Legend Biotech Corp. (LEGN)
Source Link: Johnson & Johnson Announcement
AstraZeneca PLC
Symbol: AZN
Event Phase: Approved
Drug: Tagrisso
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: Y
Molecule: Small Molecule
Target: EGFR (Epidermal Growth Factor Receptor)
LOA: 100%
Partner Companies: None
Source Link: AstraZeneca PLC Announcement
Financing events
Baseimmune
Description: Developer of a platform for pathogen analysis to create antigen and vaccine designs.
Vertical: Life Sciences
Deal Date: February 20, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised GBP 9 million of Series A venture funding from Hoxton Ventures and IQ Capital Partners to develop potential vaccine candidates.
Investors: Hoxton Ventures, IQ Capital Partners
Deal Size: GBP 9 million
Bioptimus (Seed Round)
Description: Developer of a universal AI foundation model for biology.
Verticals: Artificial Intelligence & Machine Learning, Life Sciences
Deal Date: February 19, 2024
Deal Type: Seed Round
Deal Synopsis: Raised $35 million of seed funding led by Sofinnova Partners and Bpifrance to fuel breakthrough discoveries in biomedicine.
Investors: Sofinnova Partners, Bpifrance, Hummingbird, NJF Capital, Owkin, Top Harvest Capital, Frst Capital, Cathay Innovation, Headline, Xavier Niel
Deal Size: $35 million
Desentum
Description: Developer of novel types of allergen immunotherapy products.
Vertical: Life Sciences
Deal Date: February 20, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised EUR 12 million led by Springvest to continue clinical development of allergy vaccines.
Investors: Springvest
Deal Size: EUR 12 million
Flagship Biosciences
Description: Developer of a drug development platform applying computational tissue to biomarkers.
Verticals: Life Sciences, TMT
Deal Date: February 18, 2024
Deal Type: Later Stage VC (Convertible Debt)
Deal Synopsis: Raised $3 million from undisclosed investors.
Deal Size: $3 million
Healionics
Description: Developer of clinical-stage solutions for kidney failure patients.
Verticals: Digital Health, Life Sciences
Deal Date: February 20, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $5.52 million of Series A3 funding from Keiretsu Forum and undisclosed investors.
Investors: Keiretsu Forum
Deal Size: $5.52 million
IsomAb
Description: Developer of isoform-specific antibodies to treat peripheral arterial disease.
Vertical: Life Sciences
Deal Date: February 20, 2024
Deal Type: Seed Round
Deal Synopsis: Raised GBP 7.5 million of seed funding led by Broadview Ventures to prepare for clinical trials.
Investors: Broadview Ventures, SCVC, Mercia Asset Management
Deal Size: GBP 7.5 million
Kinea Bio
Description: Operator of a biotechnology company focused on gene therapies for neuromuscular diseases.
Vertical: Life Sciences
Deal Date: February 19, 2024
Deal Type: Early Stage VC
Deal Synopsis: Raised $500,000 from Parent Project Muscular Dystrophy to address gene therapy delivery challenges.
Investors: Parent Project Muscular Dystrophy
Deal Size: $500,000
Scinvivo
Description: Developer of a medical technology platform for cancer diagnostics.
Verticals: Life Sciences, Oncology
Deal Date: February 21, 2024
Deal Type: Seed Round
Deal Synopsis: Raised EUR 4.7 million from Santec OIS Corporation, NLC, and Rijksdienst voor Ondernemend Nederland to improve imaging technology.
Investors: Santec OIS Corporation, NLC, Rijksdienst voor Ondernemend Nederland
Deal Size: EUR 4.7 million
Tagomics
Description: Developer of epigenetic-based chemicals for biomarker discovery.
Verticals: Life Sciences, Oncology
Deal Date: February 21, 2024
Deal Type: Early Stage VC
Deal Synopsis: Raised GBP 6.7 million led by Calculus Capital to support epigenetic biomarker discovery.
Investors: Calculus Capital, Illumina Ventures, Agilent Technologies, Mercia Asset Management, Meltwind Advisory, OMX Ventures, IQ Capital Partners
Deal Size: GBP 6.7 million
VRG Therapeutics
Description: Operator of a drug discovery company focused on treatments for poorly managed diseases.
Verticals: HealthTech, Life Sciences, LOHAS & Wellness
Deal Date: February 20, 2024
Deal Type: Later Stage VC
Deal Synopsis: In the process of raising $15 million of Series B funding to progress lead assets into the clinic.
Deal Size: $15 million
Reduction in force (RIF)
February 20 - Ring Therapeutics: A Flagship Pioneering spokesperson confirmed that Ring Therapeutics laid off 19 employees, representing less than 20% of the company. The news came days after another fellow Flagship biotech, Sonata Therapeutics, confirmed cutting costs as well.
Disease of the week
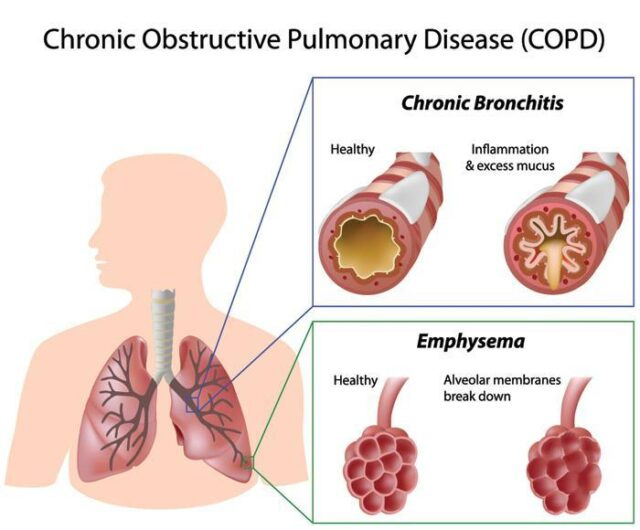
Chronic Obstructive Pulmonary Disease (COPD) is a progressive lung disease characterized by obstructed airflow from the lungs. It encompasses several conditions, primarily chronic bronchitis and emphysema, which often coexist in affected individuals. Here's a comprehensive overview of COPD:
Causes:
Smoking: The most common cause of COPD is long-term cigarette smoking. However, not all smokers develop COPD, and nonsmokers can also develop the disease.
Environmental Factors: Exposure to air pollutants, secondhand smoke, dust, chemical fumes, and other irritants over an extended period can contribute to COPD.
Genetic Factors: A small percentage of COPD cases are attributed to genetic factors, such as alpha-1 antitrypsin deficiency, a rare genetic condition.
Pathophysiology:
Airway Inflammation: Chronic inflammation in the airways leads to thickening of the bronchial walls and excess mucus production, contributing to airflow limitation.
Alveolar Destruction: Emphysema involves the destruction of lung tissue, particularly the alveoli, reducing the surface area available for gas exchange.
Airflow Limitation: The combination of inflammation, mucus production, and structural changes narrows the airways, making it difficult to exhale air from the lungs.
Symptoms:
Chronic Cough: Persistent cough with or without sputum production.
Shortness of Breath (Dyspnea): Initially with exertion but can progress to occur even at rest.
Wheezing: High-pitched whistling sounds during breathing.
Chest Tightness: Feeling of constriction or pressure in the chest.
Frequent Respiratory Infections: Due to compromised lung function.
Diagnosis:
Medical History and Physical Examination: Including symptoms, smoking history, and exposure to lung irritants.
Spirometry: Lung function test measuring the amount of air you can exhale and how quickly you can do so.
Imaging Tests: Chest X-ray or CT scan to assess lung damage and rule out other conditions.
Blood Tests: To assess oxygen levels and rule out other potential causes of symptoms.
Treatment:
Smoking Cessation: The most crucial step in halting disease progression.
Medications:
Bronchodilators: Relax muscles around the airways, improving airflow.
Inhaled Corticosteroids: Reduce airway inflammation.
Pulmonary Rehabilitation: Exercise programs, breathing techniques, and education to improve symptoms and quality of life.
Oxygen Therapy: Supplemental oxygen to alleviate hypoxemia (low blood oxygen levels).
Surgery: In severe cases, lung volume reduction surgery or lung transplantation may be considered.
Complications:
Respiratory Infections: Increased risk due to impaired lung function.
Heart Problems: COPD can strain the heart, leading to cor pulmonale (enlargement of the right side of the heart).
Lung Cancer: People with COPD have a higher risk of developing lung cancer, especially if they smoke.
Depression and Anxiety: Due to the impact of the disease on daily life and quality of life.
Prognosis:
COPD is a progressive disease with no cure, but early diagnosis and management can slow its progression, improve symptoms, and enhance quality of life. However, severe COPD can significantly reduce life expectancy, especially if not properly managed.
Prevention:
Smoking Avoidance or Cessation: The single most effective way to prevent COPD.
Minimizing Exposure to Lung Irritants: Such as air pollutants, chemical fumes, and secondhand smoke.
What I’ve read this week
*Click on the pic to read*