Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
How the market performed this week
ETFs
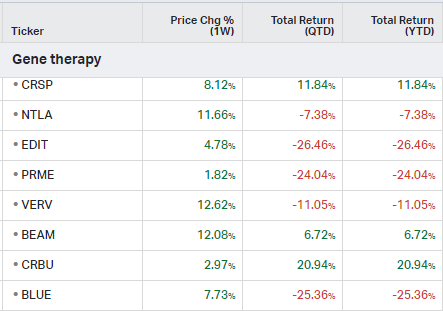
Gene Therapy
RNA Therapy
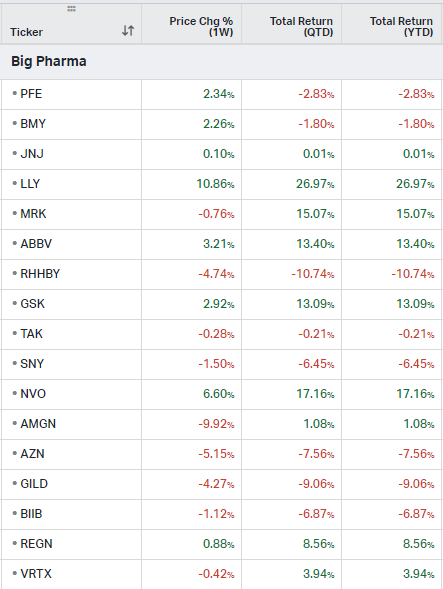
Big Pharma
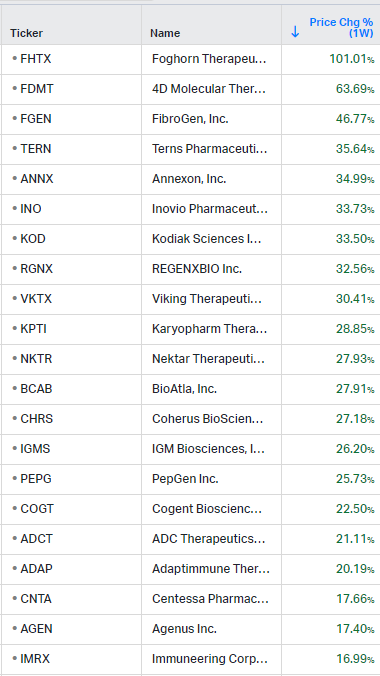
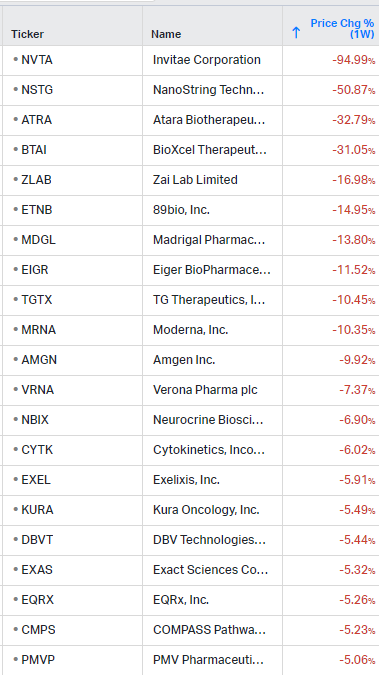
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Vicore Pharma Holding AB (VICO)
Event Phase: Preclinical
Drug: C21
Indication: Idiopathic Pulmonary Fibrosis (IPF)
Lead Indication: Y
Molecule: Small Molecule
Target: Angiotensin II Receptor Type 2 (AT2)
LOA: N/A
Partner Companies: Nippon Shinyaku Co., Ltd. (4516)
Source Link: AccessWire
Biocon, Ltd. (BIOS)
Event Phase: III
Drug: Lextemy
Indication: Renal Cell Cancer (RCC)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: VEGF (Vascular endothelial growth factor)
LOA: 44%
Partner Companies: N/A
Source Link: PRNewswire
Nicox S.A. (COX)
Event Phase: III
Drug: NCX 470
Indication: Glaucoma / Ocular Hypertension (Ophthalmology)
Lead Indication: Y
Molecule: Small Molecule
Target: Prostaglandin F receptor (FP)/PGF2 alpha receptor
LOA: 54%
Partner Companies: Kowa Company, Ltd., Ocumension Therapeutics (1477)
Source Link: GlobeNewswire
Biocon, Ltd. (BIOS)
Event Phase: Approved
Drug: Ogivri
Indication: HER2+ Breast Cancer
Lead Indication: N
Molecule: Monoclonal Antibody
Target: HER2/neu or ErbB-2
LOA: 100%
Partner Companies: N/A
Source Link: PRNewswire
Biocon, Ltd. (BIOS)
Event Phase: Approved
Drug: Ogivri
Indication: Gastric Cancer
Lead Indication: N
Molecule: Monoclonal Antibody
Target: HER2/neu or ErbB-2
LOA: 100%
Partner Companies: N/A
Source Link: PRNewswire
Biocon, Ltd. (BIOS)
Event Phase: NDA/BLA
Drug: Lextemy
Indication: Colorectal Cancer (CRC)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: VEGF (Vascular endothelial growth factor)
LOA: 92%
Partner Companies: N/A
Source Link: PRNewswire
Biocon, Ltd. (BIOS)
Event Phase: III
Drug: Lextemy
Indication: Cervical Cancer
Lead Indication: N
Molecule: Monoclonal Antibody
Target: VEGF (Vascular endothelial growth factor)
LOA: 44%
Partner Companies: N/A
Source Link: PRNewswire
Biocon, Ltd. (BIOS)
Event Phase: III
Drug: Lextemy
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Lead Indication: N
Molecule: Monoclonal Antibody
Target: VEGF (Vascular endothelial growth factor)
LOA: 44%
Partner Companies: N/A
Source Link: PRNewswire
Biocon, Ltd. (BIOS)
Event Phase: Development Outside U.S.
Drug: Lextemy
Indication: HER2+ Breast Cancer
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: VEGF (Vascular endothelial growth factor)
LOA: N/A
Partner Companies: N/A
Source Link: PRNewswire
BridgeBio Pharma, Inc. (BBIO)
Event Phase: III
Drug: Truseltiq
Indication: Achondroplasia
Lead Indication: N
Molecule: Small Molecule
Target: Fibroblast Growth Factor Receptor (FGFR)
LOA: 60%
Partner Companies: Juniper Biologics Pte Ltd, Kyowa Kirin Co., Ltd. (4151:JP), LianBio (LIAN), Novartis AG (NVS)
Source Link: GlobeNewswire
Immunome Inc. (IMNM)
Event Phase: III
Drug: AL102
Indication: Desmoid Tumors
Lead Indication: N
Molecule: Small Molecule
Target: Gamma-secretase, Notch Receptors
LOA: 45%
Partner Companies: Bristol Myers Squibb Company (BMY)
Source Link: GlobeNewswire
Clinical trials (LOA=likelihood of approval)
Hemogenyx Pharmaceuticals plc (HEMO)
Event Phase: IND
Drug: HEMO-CAR-T
Indication: Acute Myelogenous Leukemia (AML)
Lead Indication: Y
Molecule: Cellular
Target: Allogeneic Chimeric Antigen Receptor T-cells (CAR-T), Immune System
LOA: N/A
Partner Companies: N/A
Source Link: Accesswire
Roche Holding AG (RHHBY)
Event Phase: Approved
Drug: Lunsumio
Indication: Follicular Lymphoma (FL)
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Cluster of Differentiation 20 (CD20), Cluster of Differentiation 3 (CD3)
LOA: 100%
Partner Companies: Biogen, Inc. (BIIB)
Source Link: Chugai Pharm
Johnson & Johnson (JNJ)
Event Phase: II
Drug: Nipocalimab
Indication: Hemolytic Disease of the Newborn (HDN)
Lead Indication: Y
Molecule: Monoclonal Antibody
Target: Neonatal Fc Receptor
LOA: 19%
Partner Companies: N/A
Source Link: PRNewswire
BioMarin Pharmaceutical Inc. (BMRN)
Event Phase: Approved
Drug: Roctavian
Indication: Hemophilia A
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Coagulation Factor VIII, Coagulation Factor X
LOA: 100%
Partner Companies: St. Jude Children's Research Hospital, University College London
Source Link: Wiley Online Library
Athira Pharma, Inc. (ATHA)
Event Phase: Preclinical
Drug: ATH-1105
Indication: Amyotrophic Lateral Sclerosis (ALS)
Lead Indication: N
Molecule: Small Molecule
Target: Unknown
LOA: N/A
Partner Companies: N/A
Source Link: GlobeNewswire
Disc Medicine, Inc. (IRON)
Event Phase: I
Drug: DISC-3405
Indication: Polycythemia Vera (PV)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Transmembrane protease, serine 6 (Tmprss6)
LOA: 5%
Partner Companies: Mabwell Biotechnology
Source Link: GlobeNewswire
AIM Immunotech Inc. (AIM)
Event Phase: II
Drug: Ampligen
Indication: COVID-19 Treatment
Lead Indication: N
Molecule: dsRNA
Target: Immune System, Toll-like receptor 3 (TLR3)
LOA: 23%
Partner Companies: Bioclones (Pty) Ltd, Emerge Health Pty Ltd., Merck & Co., Inc. (MRK)
Source Link: GlobeNewswire
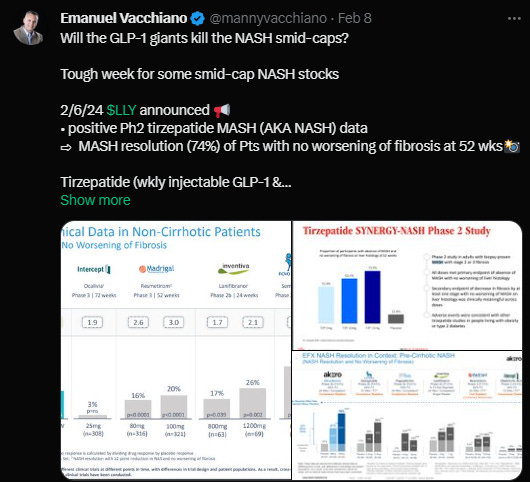
Madrigal Pharmaceuticals, Inc. (MDGL)
Event Phase: NDA/BLA
Drug: Resmetirom
Indication: Non-Alcoholic Steatohepatitis (NASH)
Lead Indication: Y
Molecule: Small Molecule
Target: Thyroid hormone receptors (TRs)
LOA: 96%
Partner Companies: Roche Holding AG (RHHBY)
Source Link: NEJM
Adverum Biotechnologies, Inc. (ADVM)
Event Phase: II
Drug: Ixo-vec
Indication: Wet Age-Related Macular Degeneration (Wet AMD) (Ophthalmology)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Vascular endothelial growth factor (VEGF)
LOA: 25%
Partner Companies: N/A
Source Link: GlobeNewswire
Corvus Pharmaceuticals, Inc. (CRVS)
Event Phase: I
Drug: Soquelitinib
Indication: Peripheral T-Cell Lymphoma (PTCL) - NHL
Lead Indication: N
Molecule: Small Molecule
Target: Interleukin-2-inducible T-cell kinase (ITK)
LOA: 5%
Partner Companies: Angel Pharmaceuticals
Source Link: GlobeNewswire
Denali Therapeutics Inc. (DNLI)
Event Phase: II
Drug: DNL126
Indication: Mucopolysaccharidosis IIIA (MPS IIIA; Sanfilippo A Syndrome)
Lead Indication: N
Molecule: Protein
Target: Sulfated alpha-L-iduronic acid
LOA: 25%
Partner Companies: N/A
Source Link: [Not provided]
Gilead Sciences, Inc. (GILD)
Event Phase: Suspended
Drug: Magrolimab
Indications: Acute Myelogenous Leukemia (AML), Diffuse Large B-Cell Lymphoma (DLBCL) - NHL, Multiple Myeloma (MM)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Cluster of Differentiation 47 (CD47), Immune System
LOA: AML - 4%, DLBCL - 1%, MM - Not specified
Partner Companies: Ono Pharmaceutical Company, Ltd. (4528:JP)
Source Link: Gilead Sciences, Inc. statement
Regenxbio Inc. (RGNX)
Event Phase: III
Drug: RGX-121
Indication: Mucopolysaccharidosis II (MPS II; Hunter Syndrome)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Iduronate-2-Sulfatase, Sulfated alpha-L-iduronic acid
LOA: 58%
Partner Companies: Not specified
Source Link: PR Newswire
BerGenBio ASA (BGBIO)
Event Phase: Development Outside U.S.
Drug: Bemcentinib
Indication: Acute Respiratory Failure, Acute Lung Injury (ALI), Acute Respiratory Distress Syndrome (ARDS)
Lead Indication: N
Molecule: Small Molecule
Target: Axl Receptor Tyrosine Kinase
LOA: Not specified
Partner Companies: Rigel Pharmaceuticals, Inc. (RIGL)
Source Link: PR Newswire
BioNxt Solutions Inc. (BNXT)
Event Phase: Preclinical
Drug: Cladribine (BioNxt)
Indication: Multiple Sclerosis (MS)
Lead Indication: N
Molecule: Small Molecule
Target: Ribonucleotide Reductase (RNR)
LOA: Not specified
Partner Companies: Not specified
Source Link: Accesswire
Johnson & Johnson (JNJ)
Event Phase: III
Drug: JNJ-2113
Indication: Psoriasis
Lead Indication: N
Molecule: Peptide
Target: Interleukin-23 (IL-23)
LOA: 64%
Partner Companies: Protagonist Therapeutics, Inc. (PTGX)
Source Link: NEJM
Gilead Sciences, Inc. (GILD)
Event Phase: Suspended
Drug: Magrolimab
Indication: Acute Myelogenous Leukemia (AML)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Cluster of Differentiation 47 (CD47), Immune System
LOA: 4%
Partner Companies: Ono Pharmaceutical Company, Ltd. (4528:JP)
Source Link: Gilead Sciences Statement
Regenxbio Inc. (RGNX)
Event Phase: II
Drug: RGX-202 (Regenxbio)
Indication: Duchenne Muscular Dystrophy (DMD)
Lead Indication: N
Molecule: Viral Gene Therapy
Target: Dystrophin gene (DMD)
LOA: 25%
Partner Companies: Not specified
Source Link: PR Newswire
Denali Therapeutics Inc. (DNLI)
Event Phase: III
Drug: DNL310
Indication: Mucopolysaccharidosis II (MPS II; Hunter Syndrome)
Lead Indication: Y
Molecule: Protein
Target: Sulfated alpha-L-iduronic acid
LOA: 58%
Partner Companies: Not specified
Source Link: PR Newswire
Gilead Sciences, Inc. (GILD)
Event Phase: III
Drug: Obeldesivir
Indication: COVID-19 Treatment
Lead Indication: Y
Molecule: Small Molecule
Target: RNA polymerase
LOA: 59%
Partner Companies: Not specified
Source Link: Business Wire
Ultragenyx Pharmaceutical Inc. (RARE)
Event Phase: II
Drug: UX111
Indication: Mucopolysaccharidosis IIIA (MPS IIIA; Sanfilippo A Syndrome)
Lead Indication: Y
Molecule: Viral Gene Therapy
Target: Heparan-N-sulfatase (N-sulphoglucosamine sulphohydrolase, sulfamidase), N-sulfo-D-glucosamine
LOA: 27%
Partner Companies: Abeona Therapeutics Inc. (ABEO)
Source Link: GlobeNewswire
UNITY Biotechnology, Inc. (UBX)
Event Phase: II
Drug: UBX1325
Indication: Diabetic Macular Edema (Ophthalmology)
Lead Indication: Y
Molecule: Small Molecule
Target: B-cell lymphoma 2 (Bcl-2)/Bcl-2 Family
LOA: 33%
Partner Companies: Ascentage Pharma Group Corporation (6855)
Source Link: GlobeNewswire
Agenus Inc. (AGEN)
Event Phase: II
Drug: agenT-797
Indication: Acute Respiratory Failure, Acute Lung Injury (ALI), Acute Respiratory Distress Syndrome (ARDS)
Lead Indication: N
Molecule: Cellular
Target: Allogeneic Chimeric Antigen Receptor T-cells (CAR-T), Immune System, T lymphocytes
LOA: 13%
Partner Companies: MiNK Therapeutics, Inc. (INKT)
Source Link: GlobeNewswire
Eli Lilly and Company (LLY)
Event Phase: II
Drug: Mounjaro
Indication: Non-Alcoholic Steatohepatitis (NASH)
Lead Indication: N
Molecule: Peptide
Target: GIP Receptor (GIPR)/Glucose-Dependent Insulinotropic Polypeptide Receptor, GLP-1 Receptor
LOA: 20%
Partner Companies: Mitsubishi Tanabe Pharma Corporation
Source Link: Lilly Investor Relations
Eli Lilly and Company (LLY)
Event Phase: III
Drug: Verzenio
Indication: Prostate Cancer
Lead Indication: N
Molecule: Small Molecule
Target: Cyclin Dependent Kinase 4 (CDK-4), Cyclin Dependent Kinase 6 (CDK-6)
LOA: 44%
Partner Companies: Not specified
Source Link: Lilly Investor Relations
Gain Therapeutics, Inc. (GANX)
Event Phase: Development Outside U.S.
Drug: GT-02287
Indication: Parkinson's Disease (PD)
Lead Indication: N
Molecule: Small Molecule
Target: GBA2 (extralysosomal glucocerebrosidase)
LOA: Not specified
Partner Companies: Not specified
Source Link: GlobeNewswire
GeoVax Labs, Inc. (GOVX)
Event Phase: II
Drug: GEO-CM04S1
Indication: COVID-19 Prevention
Lead Indication: Y
Molecule: Vaccine
Target: Immune System, SARS-CoV-2
LOA: 23%
Partner Companies: City of Hope
Source Link: GlobeNewswire
Arcturus Therapeutics Ltd. (ARCT)
Event Phase: II
Drug: ARCT-154
Indication: COVID-19 Prevention
Lead Indication: N
Molecule: Vaccine
Target: SARS-CoV-2
LOA: 23%
Partner Companies: Not specified
Source Link: BusinessWire
Johnson & Johnson (JNJ)
Event Phase: II
Drug: Nipocalimab
Indication: Sjogren's Syndrome
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Neonatal Fc Receptor
LOA: 19%
Partner Companies: Not specified
Source Link: PR Newswire
Johnson & Johnson (JNJ)
Event Phase: III
Drug: Nipocalimab
Indication: Myasthenia Gravis (MG)
Lead Indication: N
Molecule: Monoclonal Antibody
Target: Neonatal Fc Receptor
LOA: 64%
Partner Companies: Not specified
Source Link: PR Newswire
Rani Therapeutics Holdings, Inc. (RANI)
Event Phase: Development Outside U.S.
Drug: RT-111
Indication: Psoriasis
Lead Indication: N
Molecule: Not Specified
Target: Unknown
LOA: Not specified
Partner Companies: Celltrion, Inc. (068270)
Source Link: GlobeNewswire
Financing events
Immix Biopharma (NAS: IMMX)
Description: Clinical-stage biopharmaceutical company developing cell therapies for hematologic malignancies.
Verticals: Life Sciences, Oncology
Deal Date: 08-févr-2024
Deal Type: Public Investment 2nd Offering
Investors: Not specified
Deal Size: $15.00 million
Jasper Therapeutics (NAS: JSPR)
Description: Clinical-stage biotechnology company focused on enabling cures through hematopoietic stem cell therapy.
Verticals: Life Sciences, Oncology
Deal Date: 07-févr-2024
Deal Type: Public Investment 2nd Offering
Investors: Not specified
Deal Size: $50.51 million
Kyverna (NAS: KYTX)
Description: Clinical-stage biopharmaceutical company developing cell therapies for autoimmune diseases.
Verticals: Life Sciences
Deal Date: 08-févr-2024
Deal Type: IPO
Investors: Not specified
Deal Size: $319.00 million
Mineralys (NAS: MLYS)
Description: Clinical-stage biopharmaceutical company focused on developing medicines targeting diseases driven by elevated aldosterone.
Verticals: Life Sciences
Deal Date: 08-févr-2024
Deal Type: PIPE
Investors: Venrock, OrbiMed, TCG Crossover Management, Samsara BioCapital, RA Capital Management, and others
Deal Size: $120.00 million
Model Medicines
Description: Developer of an AI-driven drug discovery platform using artificial intelligence to accelerate drug creation.
Verticals: Artificial Intelligence & Machine Learning, HealthTech, Life Sciences, Oncology
Deal Date: 06-févr-2024
Deal Type: Early Stage VC
Investors: Undisclosed
Deal Size: $0.95 million
myBIOS
Description: Producer of protein-based biological molecules for recombinant production of natural biomaterials and enzymes.
Verticals: Life Sciences
Deal Date: 08-févr-2024
Deal Type: Early Stage VC
Investors: Sparkalis
Deal Size: Undisclosed
Neurona Therapeutics
Description: Operator of a clinical-stage biotherapeutics company developing regenerative neural cell therapies for chronic neurological disorders.
Verticals: Life Sciences, Oncology
Deal Date: 08-févr-2024
Deal Type: Later Stage VC
Investors: Viking Global Investors, Cormorant Asset Management, and others
Deal Size: $120.00 million
NexImmune (NAS: NEXI)
Description: Clinical-stage biotechnology company developing immunotherapy for cancer and immune-mediated diseases.
Verticals: Life Sciences, Nanotechnology, Oncology, TMT
Deal Date: 06-févr-2024
Deal Type: Public Investment 2nd Offering
Investors: Not specified
Deal Size: $1.41 million
PepGen (NAS: PEPG)
Description: Clinical-stage biotechnology company advancing oligonucleotide therapeutics for severe neuromuscular and neurologic diseases.
Verticals: HealthTech, Life Sciences
Deal Date: 07-févr-2024
Deal Type: Public Investment 2nd Offering
Investors: Not specified
Deal Size: $80.08 million
Procyrion
Description: Operator of a medical device company developing catheter-based circulatory support technologies for heart failure and kidney disease patients.
Verticals: Life Sciences
Deal Date: 05-févr-2024
Deal Type: Later Stage VC
Investors: Fannin Innovation Studio, Bluebird Ventures, and others
Deal Size: $57.70 million
Redx Pharma (Kirsten Rat Sarcoma Virus Inhibitor Program in Macclesfield, United Kingdom)
Description: Developer of inhibitors for colorectal, pancreatic, and lung cancer patients.
Verticals: Life Sciences, Oncology
Deal Date: 07-févr-2024
Deal Type: Merger/Acquisition
Acquirer: Jazz Pharmaceuticals (NAS: JAZZ)
Deal Size: Undisclosed
Revelation BioSciences (NAS: REVB)
Description: Clinical-stage life sciences company focused on developing immunologic-based therapies for disease prevention and treatment.
Verticals: Life Sciences
Deal Date: 05-févr-2024
Deal Type: Public Investment 2nd Offering
Investors: Not specified
Deal Size: $0.58 million
Sartorius Stedim Biotech (PAR: DIM)
Description: Provider of bioprocessing solutions for manufacturing biologic drugs.
Verticals: Life Sciences, Manufacturing
Deal Date: 07-févr-2024
Deal Type: PIPE
Investors: Sartorius
Deal Size: EUR 3.5 billion
Seranova Bio
Description: Developer of immunotherapies for cancer treatment.
Verticals: Life Sciences, Oncology
Deal Date: 08-févr-2024
Deal Type: Early Stage VC
Investors: AbbVie (NYSE: ABBV)
Deal Size: $69.00 million
Silence Therapeutics (PINX: SLNCF)
Description: Developer of RNA interference therapeutics for rare diseases.
Verticals: HealthTech, Life Sciences
Deal Date: 05-févr-2024
Deal Type: PIPE
Investors: Novo Holdings, RTW Investments
Deal Size: $175.00 million
SwiftSync
Description: Developer of cloud-based software for medical device companies.
Verticals: HealthTech, Life Sciences, Software
Deal Date: 06-févr-2024
Deal Type: Later Stage VC
Investors: Edison Partners
Deal Size: $15.00 million
Vaccinex (NAS: VCNX)
Description: Clinical-stage biotechnology company developing immunotherapy treatments for cancer and neurodegenerative diseases.
Verticals: HealthTech, Life Sciences, Oncology
Deal Date: 08-févr-2024
Deal Type: PIPE
Investors: Bain Capital Life Sciences, Perceptive Advisors, venBio Partners, BlackRock
Deal Size: $20.00 million
Reduction in force (RIF)
February 9 - Roche: The Swiss drug developer plans to lay off a few hundred people in its product development team. Roche says it expects “the majority of the proposed headcount reduction to impact external contractors,” with less than 6% of the permanent product development team potentially impacted. Story
February 9 - Synlogic: The Massachusetts-based biotech is discontinuing a pivotal phase 3 trial, shuttering operations and laying off more than 90% of its workforce as it undergoes a strategic review.Story
February 8 - Sandoz: 20 years ago, Sandoz carved out a sizable generics foothold through dual mergers with Germany's Hexal and U.S.-based Eon Labs. Now, the Swiss manufacturing giant is permanently closing one of the latter company's main facilities in Wilson, North Carolina, with 213 employees set to be laid off by Oct. 4. Story
February 6 - Rallybio: The biotech is parting ways with 45% of its staff, or 19 employees, and focusing on its two phase 2-ready programs to extend its cash runway into the middle of 2026. Story
Disease of the week
Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system (CNS), which includes the brain and spinal cord. In MS, the immune system attacks the protective covering of nerve fibers called myelin, resulting in inflammation, damage to the myelin, and sometimes to the underlying nerves.
Here are some key points about multiple sclerosis:
Symptoms: The symptoms of MS can vary widely from person to person and depend on which nerves are affected and to what extent. Common symptoms include fatigue, numbness or weakness in one or more limbs, tremors, problems with coordination and balance, blurred or double vision, slurred speech, and cognitive impairment.
Types of MS: There are several types of MS, including:
Relapsing-remitting MS (RRMS): This is the most common form, characterized by periods of symptom exacerbation (relapses) followed by periods of partial or complete recovery (remissions).
Primary progressive MS (PPMS): In this type, symptoms gradually worsen over time without distinct relapses or remissions.
Secondary progressive MS (SPMS): This follows an initial relapsing-remitting course, with later progression of disability.
Progressive-relapsing MS (PRMS): This is characterized by a steadily worsening disease course with occasional acute relapses, without full recovery from the relapses.
Causes: The exact cause of MS is unknown, but it is believed to involve a combination of genetic, environmental, and immunological factors. Factors such as genetics, infections, vitamin D deficiency, and smoking have been implicated in increasing the risk of developing MS.
Diagnosis: There is no single test to diagnose MS. Diagnosis is based on medical history, neurological exams, MRI scans to detect lesions in the CNS, and sometimes other tests such as lumbar puncture (spinal tap) to analyze cerebrospinal fluid.
Treatment: While there is currently no cure for MS, there are treatments aimed at managing symptoms, slowing disease progression, and reducing the frequency and severity of relapses. Medications such as corticosteroids, immunomodulators, and monoclonal antibodies may be prescribed depending on the type and severity of the disease. Physical therapy, occupational therapy, and other forms of rehabilitation can also help manage symptoms and improve quality of life.
Prognosis: The course of MS varies greatly among individuals. Some people may experience relatively mild symptoms and periods of remission, while others may have severe disability and progressive decline in function. Life expectancy is generally shortened compared to the general population, but many people with MS live relatively normal lives with proper management and support.
Research and Future Directions: Research into MS continues to explore potential causes, risk factors, and treatments. Advances in understanding the immune system and the role of inflammation in MS have led to the development of new therapies, and ongoing clinical trials are investigating novel treatments aimed at stopping disease progression and promoting repair of damaged myelin.
What I’ve read this week
*Click on the pic to read*