Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
How the market performed this week
ETFs
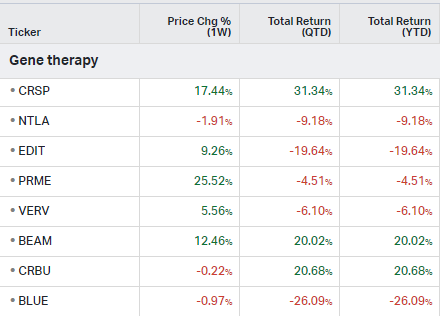
Gene Therapy
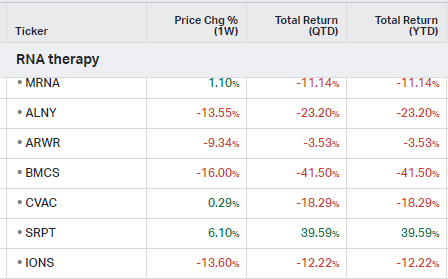
RNA Therapy
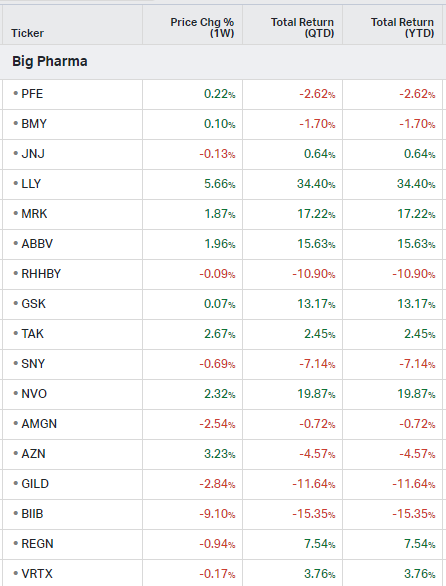
Big Pharma
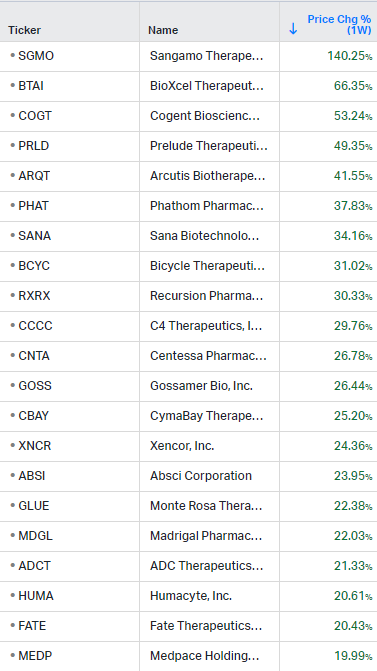
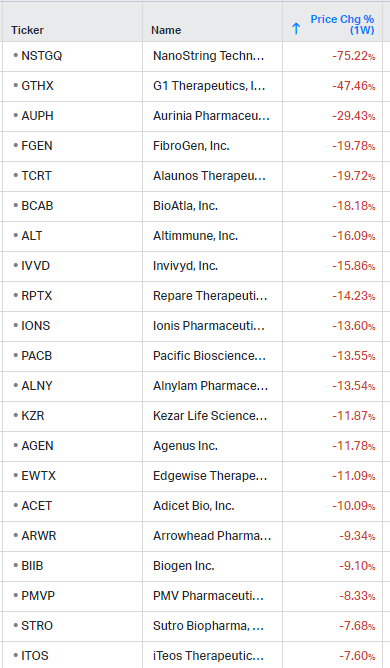
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Kinnate Biopharma, Inc. (KNTE)
Lead Company: Kinnate Biopharma, Inc.
Symbol: KNTE
Event Phase: I (Exarafenib, KIN-3248), IND (KIN-7136), Preclinical (KIN-8741, KIN004)
Drug/ Molecule: Exarafenib, KIN-3248, KIN-7136, KIN-8741, KIN004
Indication/ Lead Indication: Solid Tumors (Exarafenib, KIN-3248, KIN-7136, KIN-8741, KIN004), Biliary Tract Cancer (KIN-3248)
Target: Raf kinase (Exarafenib, KIN-3248), Fibroblast Growth Factor Receptor (FGFR) (KIN-3248), Mitogen-activated ERK kinase (MEK, MAPKK, MAP2K) (KIN-7136), Hepatocyte growth factor receptor (c-Met, HGFR) (KIN-8741), Cyclin Dependent Kinase 12 (CDK-12) (KIN004)
LOA: 5% (Exarafenib, KIN-3248, KIN-7136, KIN004)
Partner Companies: Kinnjiu Biopharma Inc.
Source Link: Kinnate Biopharma Inc. Enters into Agreement to be Acquired by XOMA Corporation
GSK plc (GSK)
Lead Company: GSK plc
Symbol: GSK
Event Phase: Development Outside U.S.
Drug/ Molecule: SHR-1905
Indication/ Lead Indication: Chronic Rhinosinusitis
Target: Thymic stromal lymphopoeitin (TSLP)
Partner Companies: Jiangsu Hengrui Pharmaceuticals
Source Link: GSK Completes Acquisition of Aiolos Bio
Harrow, Inc. (HROW)
Lead Company: Harrow, Inc.
Symbol: HROW
Event Phase: Approved
Drugs/ Molecules: Iheezo, Verkazia, VEVYE, Zerviate
Indication/ Lead Indication: Anesthesia (Iheezo), Allergic Conjunctivitis (Ophthalmology) (Verkazia, Zerviate), Dry Eye (Ophthalmology) (VEVYE)
Target: Unknown (Iheezo), Calcineurin phosphatase (Verkazia), Cyclophilin (VEVYE), Histamine H1 Receptor (HRH1) (Zerviate)
LOA: 100%
Partner Companies: Apotex Inc., Sintetica SA, Santen Pharmaceutical Co., Ltd., Novaliq GmbH, Hikma Pharmaceuticals plc, ITROM Pharmaceutical Group, Lupin Limited, Nicox S.A., Ocumension Therapeutics, Samil Pharmaceutical Co., Ltd.
Source Link: Harrow Licenses Canadian Rights to Apotex for Five Branded Ophthalmic Pharmaceutical Products
Bristol Myers Squibb Company (BMY)
Lead Company: Bristol Myers Squibb Company
Symbol: BMY
Event Phase: II
Drug/ Molecule: BMS-986392
Indication/ Lead Indication: Solid Tumors
Target: HER2/neu or ErbB-2, Natural Killer Cells (NK Cells)
LOA: 11%
Partner Companies: Dragonfly Therapeutics, Inc.
Erasca, Inc. (ERAS)
Lead Company: Erasca, Inc.
Symbol: ERAS
Event Phase: II (LXH254), I (LXH254)
Drug/ Molecule: LXH254
Indication/ Lead Indication: Melanoma (LXH254), Solid Tumors (LXH254)
Target: Raf kinase
LOA: 11% (LXH254), 5% (LXH254)
Partner Companies: Novartis AG
CERo Therapeutics Holdings, Inc (CERO)
Lead Company: CERo Therapeutics Holdings, Inc
Symbol: CERO
Event Phase: Preclinical
Drug/ Molecule: CER-1236
Indication/ Lead Indication: Hematologic Cancer
Target: Immune System, Stem Cells/Other Cell Therapies
Source Link: Phoenix Biotech Acquisition Corp. and CERo Therapeutics Inc. Announce Close of Business Combination
Coya Therapeutics, Inc. (COYA)
Lead Company: Coya Therapeutics, Inc.
Symbol: COYA
Event Phase: Preclinical
Drug/ Molecule: COYA-301
Indication/ Lead Indication: Inflammatory Disorders
Target: Unknown
Partner Companies: ARScience Biotherapeutics, Inc., Dr. Reddy's Laboratories Ltd.
Source Link: Business Wire
Johnson & Johnson (JNJ)
Lead Company: Johnson & Johnson
Symbol: JNJ
Event Phase: III
Drug/ Molecule: Botaretigene sparoparvovec
Indication/ Lead Indication: Retinitis Pigmentosa (RP) (Ophthalmology)
Target: Retinitis Pigmentosa GTPase Regulator (RPGR)
LOA: 54%
Source Link: MeiraGTx Announces $50 Million Milestone from Janssen Pharmaceuticals
Lantheus Holdings, Inc. (LNTH)
Lead Company: Lantheus Holdings, Inc.
Symbol: LNTH
Event Phase: I
Drug/ Molecule: MK-6240
Indication/ Lead Indication: Alzheimer's Disease - Imaging
Target: Tau proteins
LOA: 6%
Partner Companies: Alector Inc., Lexeo Therapeutics, Merck & Co., Inc., Prothena Corporation plc, Sinotau Pharmaceuticals Group
CymaBay Therapeutics, Inc. (CBAY)
Lead Company: CymaBay Therapeutics, Inc.
Symbol: CBAY
Event Phase: NDA/BLA
Drug/ Molecule: Seladelpar
Indication/ Lead Indication: Primary Biliary Cholangitis (PBC)
Target: PPAR delta
LOA: 99%
Partner Companies: Johnson & Johnson, Kaken Pharmaceutical Company, Ltd
Source Link: Business Wire
AbbVie Inc. (ABBV)
Lead Company: AbbVie Inc.
Symbol: ABBV
Event Phase: Approved (Elahere, Sarclisa), II (SAR408701, Pivekimab), I (IMGC936, IMGN151), Development Outside U.S. (SAR408701)
Drugs/ Molecules: Elahere, Sarclisa, SAR408701, Pivekimab, IMGC936, IMGN151
Indication/ Lead Indication: Ovarian Cancer (Elahere), Multiple Myeloma (MM) (Sarclisa), Solid Tumors (SAR408701, IMGC936), Acute Lymphoblastic Leukemia (ALL) (Pivekimab), Acute Myelogenous Leukemia (AML) (Pivekimab), Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) (Pivekimab), Cancer (IMGN151)
Target: Folate Receptor (FOLR1), Microtubules (Tubulin) (Elahere), Cluster of Differentiation 38 (CD38), Immune System (Sarclisa), CEACAM5 (CD66e) (SAR408701), IL-3 (Interleukin-3) Receptor/CD123, Immune System (Pivekimab), A Disintegrin and Metalloproteinase 9 (ADAM9), Immune System (IMGC936), Folate Receptor (FOLR1) (IMGN151)
LOA: 100% (Elahere, Sarclisa), 11% (SAR408701, Sarclisa), 5% (IMGC936, IMGN151)
Partner Companies: Huadong Medicine Co., Ltd., Takeda Pharmaceutical Co. Ltd., AbbVie Inc. (for various drugs)
Source Link: AbbVie Completes Acquisition of Immunogen
Sanofi (SNY)
Lead Company: Sanofi
Symbol: SNY
Event Phase: Approved (Sarclisa), II (SAR408701, Sarclisa), Development Outside U.S. (SAR408701)
Drugs/ Molecules: Sarclisa, SAR408701
Indication/ Lead Indication: Multiple Myeloma (MM) (Sarclisa), Solid Tumors (SAR408701)
Target: Cluster of Differentiation 38 (CD38), Immune System (Sarclisa), CEACAM5 (CD66e) (SAR408701)
LOA: 100% (Sarclisa), 11% (SAR408701, Sarclisa)
Partner Companies: AbbVie Inc. (for various drugs), Innovent Biologics, Inc.
Source Link: AbbVie Completes Acquisition of Immunogen
Takeda Pharmaceutical Co. Ltd. (TAK)
Lead Company: Takeda Pharmaceutical Co. Ltd.
Symbol: TAK
Event Phase: I
Drug/ Molecule: TAK-164
Indication/ Lead Indication: Colorectal Cancer (CRC)
Target: Guanylate Cyclase (sGC), Immune System
LOA: 5%
Partner Companies: AbbVie Inc.
Source Link: AbbVie Completes Acquisition of Immunogen
CytomX Therapeutics, Inc. (CTMX)
Lead Company: CytomX Therapeutics, Inc.
Drug: CX-2009
Indication: HR+/HER2- Breast Cancer, Triple-Negative Breast Cancer (TNBC)
Target: Cluster of Differentiation 166 (CD166) / ALCAM, Microtubules (Tubulin)
Phase: Phase II
LOA: 11%
Partner Company: AbbVie Inc. (ABBV)
Source Link: AbbVie Completes Acquisition of Immunogen
Sanofi (SNY)
Lead Company: Sanofi
Drug: Sarclisa
Indication: Solid Tumors
Target: Cluster of Differentiation 38 (CD38), Immune System
Phase: Phase II
LOA: 11%
Partner Company: AbbVie Inc. (ABBV)
Source Link: AbbVie Completes Acquisition of Immunogen
Takeda Pharmaceutical Co. Ltd. (TAK)
Lead Company: Takeda Pharmaceutical Co. Ltd.
Drug: TAK-164
Indication: Colorectal Cancer (CRC)
Target: Guanylate Cyclase (sGC), Immune System
Phase: Phase I
LOA: 5%
Partner Company: AbbVie Inc. (ABBV)
Source Link: AbbVie Completes Acquisition of Immunogen
Sanofi (SNY)
Lead Company: Sanofi
Drug: SAR408701
Indication: Gastric Cancer
Target: CEACAM5 (CD66e)
Phase: Development Outside U.S.
Partner Companies: AbbVie Inc. (ABBV), Innovent Biologics, Inc. (1801)
Source Link: AbbVie Completes Acquisition of Immunogen
Repare Therapeutics, Inc. (RPTX)
Lead Company: Repare Therapeutics, Inc.
Drug: Camonsertib
Indication: Solid Tumors
Target: Ataxia Telangiectasia and Rad3-related Protein (ATR)/Frap-related Protein 1 (FRP1)
Phase: Phase II
LOA: 11%
Source Link: Repare Therapeutics to Regain Global Rights to Camonsertib
Clinical trials (LOA=likelihood of approval)
Iovance Biotherapeutics, Inc. (IOVA)
Lead Company: Iovance Biotherapeutics, Inc.
Symbol: IOVA
Event: Approved
Drug: Amtagvi
Indication: Melanoma
Lead Indication: Melanoma
Molecule: Cellular
Target: Immune System, Stem Cells/Other Cell Therapies, T lymphocytes
LOA: 105%
Partner Companies: None
Source Link: Iovance's AMTAGVI lifileucel Receives U.S. FDA Accelerated Approval for Advanced Melanoma
Sanofi (SNY)
Lead Company: Sanofi
Symbol: SNY
Event: Phase III
Drug: SAR441344
Indication: Multiple Sclerosis (MS)
Molecule: Monoclonal Antibody
Target: Cluster of Differentiation 154 (CD154) / CD40 Ligand (CD40L) / gp39
LOA: 48%
Partner Companies: ImmuNext Inc.
Source Link: NEJMoa2309439
Applied Therapeutics Inc. (APLT)
Lead Company: Applied Therapeutics Inc.
Symbol: APLT
Event: Phase III
Drug: AT-007
Indication: Neurology - Other
Molecule: Small Molecule
Target: Aldose Reductase
LOA: 50%
Partner Companies: ADVANZ PHARMA Corp. Limited
Ensysce Biosciences, Inc. (ENSC)
Lead Company: Ensysce Biosciences, Inc.
Symbol: ENSC
Event: Phase II
Drug: PF-614
Indication: Moderate to Severe Pain
Molecule: Small Molecule
Target: Opioid receptors
LOA: 12%
Partner Companies: None
Source Link: Ensysce Biosciences Announces Publication of Clinical Bioequivalence Manuscript
Galapagos NV (GLPG)
Lead Company: Galapagos NV
Symbol: GLPG
Event: Development Outside U.S.
Drug: GLPG5201
Indication: Chronic Lymphocytic Leukemia (CLL)/Small Cell Lymphocytic Lymphoma (SLL) - NHL
Molecule: Cellular
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), Cluster of Differentiation 19 (CD19)
LOA: Not specified
Partner Companies: None
Source Link: Galapagos presents at EBMT EHA annual meeting 2024
Corcept Therapeutics Incorporated (CORT)
Lead Company: Corcept Therapeutics Incorporated
Symbol: CORT
Event: Approved
Drug: Korlym
Indication: Cushing's Syndrome
Molecule: Small Molecule
Target: Androgen receptors, Glucocorticoid Receptor (GR), Progesterone Receptor
LOA: 100%
Partner Companies: None
Source Link: Corcept Announces Preliminary Results From Prevalence Phase Of CATALYST Clinical Trial
Galapagos NV (GLPG)
Lead Company: Galapagos NV
Symbol: GLPG
Event: Development Outside U.S.
Drug: GLPG5101
Indication: Non-Hodgkin's Lymphoma (NHL)
Molecule: Cellular
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), Cluster of Differentiation 19 (CD19)
LOA: Not specified
Partner Companies: None
Source Link: Galapagos presents at EBMT EHA annual meeting 2024
Cardiol Therapeutics Inc. (CRDL)
Lead Company: Cardiol Therapeutics Inc.
Symbol: CRDL
Event: Phase II
Drug: Oral CardiolRx
Indication: Myocarditis
Molecule: Small Molecule
Target: Adenosine Receptors, Cannabinoid-1 (CB1) receptor, GPR55, TRPV1, transient receptor potential vanilloid receptor 1, VR-1
LOA: 10%
Partner Companies: None
Source Link: Cardiol Therapeutics Granted Orphan Drug Designation for its Lead Drug Candidate for the Treatment of Pericarditis
Merck KGaA (MKKGY)
Lead Company: Merck KGaA
Symbol: MKKGY
Event: Approved
Drug: Tepmetko
Indication: Non-Small Cell Lung Cancer (NSCLC)
Molecule: Small Molecule
Target: Hepatocyte growth factor receptor (c-Met, HGFR)
LOA: 100%
Partner Companies: None
Source Link: FDA Approves Tepotinib for Metastatic Non-Small Cell Lung Cancer
Biotest AG (BIO:GR)
Lead Company: Biotest AG
Symbol: BIO:GR
Event: Development Outside U.S.
Drug: BT524
Indication: Hemostasis
Molecule: Protein
Target: Fibrinogen (Coagulation Factor I)
LOA: 3%
Partner Companies: None
Source Link: Grifols announces positive topline phase 3 fibrinogen clinical trial results
Kiromic BioPharma, Inc. (KRBP)
Lead Company: Kiromic BioPharma, Inc.
Symbol: KRBP
Event: Phase I
Drug: Deltacel
Indication: Non-Small Cell Lung Cancer (NSCLC)
Molecule: Cellular
Target: Unknown
LOA: 5%
Partner Companies: None
Source Link: Press Release
Silo Pharma, Inc. (SILO)
Lead Company: Silo Pharma, Inc.
Symbol: SILO
Event: Preclinical
Drug: SP-26
Indication: Fibromyalgia
Molecule: Small Molecule
Target: NMDA Glutamate Receptor
LOA: Not specified
Partner Companies: Zylö Therapeutics
Source Link: Silo Pharma's SP-26 Ketamine Implant Demonstrates Successful Drug Delivery
Annovis Bio, Inc. (ANVS)
Lead Company: Annovis Bio, Inc.
Symbol: ANVS
Event: Phase III
Drug: Buntanetap
Indication: Alzheimer's Disease (AD)
Molecule: Small Molecule
Target: Alpha-synuclein, Amyloid Beta/Amyloid Plaques, B-APP Synthesis, Tau proteins
LOA: 46%
Partner Companies: None
Source Link: Annovis Bio Announces Last Patient Last Visit in the Phase II/III Study of Buntanetap in Alzheimer's Disease
Ampio Pharmaceuticals, Inc. (AMPE)
Lead Company: Ampio Pharmaceuticals, Inc.
Symbol: AMPE
Event: Preclinical
Drug: OA-201
Indication: Osteoarthritis Pain
Molecule: Small Molecule
Target: Unknown
LOA: Not specified
Partner Companies: None
Source Link: Ampio Provides Update on Results from Pre-IND Enabling Studies
Vaxxinity, Inc. (VAXX)
Lead Company: Vaxxinity, Inc.
Symbol: VAXX
Event: Development Outside U.S.
Drug: VXX-401
Indication: Dyslipidemia / Hypercholesterolemia
Molecule: Peptide
Target: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9)
LOA: Not specified
Partner Companies: None
Source Link: Vaxxinity's Cholesterol Vaccine Candidate Successfully Lowers LDL-C Preclinical Data Published
RespireRx Pharmaceuticals Inc. (RSPI)
Lead Company: RespireRx Pharmaceuticals Inc.
Symbol: RSPI
Event: Preclinical
Drug: KRM-II-81
Indication: Pain Indications
Molecule: Small Molecule
Target: GABA-A Receptor
LOA: Not specified
Partner Companies: University of Wisconsin, Madison
Source Link: RespireRx Pharmaceuticals Inc. Reports Preclinical Pain Relief for their Non-Opioid Lead GABAkine
KalVista Pharmaceuticals, Inc. (KALV)
Lead Company: KalVista Pharmaceuticals, Inc.
Symbol: KALV
Event: Phase III
Drug: Sebetralstat
Indication: Hereditary Angioedema (HAE)
Molecule: Small Molecule
Target: Kinin-Kallikrein System
LOA: 70%
Partner Companies: None
Source Link: Press Release
Satellos Bioscience, Inc. (MSCL)
Lead Company: Satellos Bioscience, Inc.
Symbol: MSCL
Event: Preclinical
Drug: SAT-3247
Indication: Duchenne Muscular Dystrophy (DMD)
Molecule: Small Molecule
Target: Adaptor-Associated Kinase 1 (AAK1)
LOA: Not specified
Source Link: Business Wire
Incyte Corporation (INCY)
Lead Company: Incyte Corporation
Symbol: INCY
Event: Phase I
Drug: INCB123667
Indication: Solid Tumors
Molecule: Small Molecule
Target: Cyclin Dependent Kinase 2 (CDK-2)
LOA: 5%
Source Link: Incyte Reports 2023 Fourth Quarter and Year-End Financial
Larimar Therapeutics, Inc. (LRMR)
Lead Company: Larimar Therapeutics, Inc.
Symbol: LRMR
Event: Phase II
Drug: Nomlabofusp
Indication: Friedreich's Ataxia
Molecule: Protein
Target: Frataxin (FXN), Mitochondria
LOA: 12%
Harmony Biosciences Holdings, Inc. (HRMY)
Lead Company: Harmony Biosciences Holdings, Inc.
Symbol: HRMY
Event: Phase II
Drug: Wakix
Indication: Prader-Willi Syndrome
Molecule: Small Molecule
Target: Histamine H3 Receptor (HRH3)
LOA: 17%
Partner Companies: AOP Orphan Pharmaceuticals AG, BioProjet SCR, Endo International plc (ENDP), Grupo Ferrer Internacional, S.A.
Source Link: FDA Approved Drug Products: Wakix for Prader-Willi Syndrome
Financing events
Alys Pharmaceuticals
Description: Developer of an immuno-dermatology platform designed to revolutionize treatment paradigms across various dermatological indications.
Verticals: Digital Health, Life Sciences
Deal Date: 12-févr-2024
Deal Type: Seed Round
Deal Synopsis: The company raised $100 million of Seed funding from Medicxi on February 12, 2024, putting the company's pre-money valuation at $50 million.
Investors: Medicxi (Francesco De Rubertis)
Deal Size: $100.00
Areteia Therapeutics
Description: Developer of oral drug intended to help patients with eosinophilic asthma.
Verticals: Life Sciences
Deal Date: 13-févr-2024
Deal Type: Early Stage VC
Deal Synopsis: The company raised $425 million of Series A-1 venture funding in a deal led by Bain Capital Life Sciences on February 13, 2024, putting the company's pre-money valuation at $695 million.
Investors: Access Biotechnology, ARCH Venture Partners (Paul Berns), Bain Capital Life Sciences (Adam Koppel), GV, Marshall Wace, Maverick Capital, Population Health Partners, Sanofi (PAR: SAN), Saturn Partners, Viking Global Investors
Deal Size: $425.00
Autolus (NAS: AUTL)
Description: Autolus Therapeutics PLC is a biopharmaceutical company developing next-generation programmed T-cell therapies for the treatment of cancer.
Verticals: Life Sciences, Oncology
Deal Date: 12-févr-2024
Deal Type: PIPE
Deal Synopsis: The company (NAS:AUTL) received $200 million of development capital from BioNTech on February 12, 2024 through a private placement.
Investors: BioNTech (NAS: BNTX) (Ugur Sahin)
Deal Size: $200.00
Cagent Vascular
Description: Developer of a next-generation intravascular technology designed to treat diseased peripheral arteries.
Verticals: Life Sciences
Deal Date: 14-févr-2024
Deal Type: Later Stage VC
Deal Synopsis: The company raised $28.74 million of venture funding from undisclosed investors on February 14, 2024.
Deal Size: $28.74
Cogent Biosciences (NAS: COGT)
Description: Cogent Biosciences Inc a biotechnology company focused on developing precision therapies for genetically defined diseases.
Verticals: Life Sciences, Oncology
Deal Date: 14-févr-2024
Deal Type: PIPE
Deal Synopsis: The company (NAS:COGT) is in talks to receive $225 million of development capital from Fairmount Funds Management, Venrock, Janus Cycle Group, Deerfield Agency, Commodore Capital, TCG Crossover Management, Adage Capital Management, Redmile Group and Perceptive Advisors through a private placement as of February 14, 2024.
Investors: Adage Capital Management, Commodore Capital, Deerfield Agency, Fairmount Funds Management (Peter Harwin), Janus Cycle Group, Perceptive Advisors, Redmile Group, TCG Crossover Management, Venrock
Deal Size: $225.00
CRISPR Therapeutics (NAS: CRSP)
Description: CRISPR Therapeutics is a gene editing company focused on the development of CRISPR/Cas9-based therapeutics.
Verticals: Life Sciences, TMT
Deal Date: 13-févr-2024
Deal Type: PIPE
Deal Synopsis: The company (NAS:CRSP) is in talks to receive $280 million of development capital from undisclosed investors through a private placement as of February 13, 2024.
Deal Size: $280.00
CymaBay Therapeutics (NAS: CBAY)
Description: CymaBay Therapeutics Inc is a clinical-stage biopharmaceutical company focused on developing innovative therapies for patients with liver and other chronic diseases.
Verticals: Life Sciences
Deal Date: 12-févr-2024
Deal Type: Merger/Acquisition
Deal Synopsis: The company reached a definitive agreement to be acquired by Gilead Sciences (NAS: GILD) for $4.3 billion on February 12, 2024.
Investors: Gilead Sciences (NAS: GILD) (Daniel O'Day)
Deal Size: $4 300.00
enGene
Description: Operator of a late-stage biotechnology company focused on mainstreaming genetic medicines through the delivery of therapeutics to mucosal tissues and other organs.
Verticals: HealthTech, Life Sciences
Deal Date: 14-févr-2024
Deal Type: PIPE
Deal Synopsis: The company is in talks to receive CAD 200 million of development capital from Boxer Capital, Venrock, Soleus Capital, Deep Track Capital, Foresite Capital, Commodore Capital, Lumira Ventures, Adage Capital Management, Cormorant Asset Management, Janus Henderson Investors, Logos Capital, Marshall Wace, Perceptive Advisors, Surveyor Capital and Blue Owl Capital through a private placement as of February 14, 2024.
Investors: Adage Capital Management, Blue Owl Capital (NYS: OWL), Boxer Capital, Commodore Capital, Cormorant Asset Management, Deep Track Capital, Foresite Capital, Janus Henderson Investors (NYS: JHG), Logos Capital, Lumira Ventures, Marshall Wace, Perceptive Advisors, Soleus Capital, Surveyor Capital, Venrock
Deal Size: CAD 200.00
FireFly Bio
Description: Operator of an antibody-drug conjugates-based proprietary healthcare platform intended to treat cancer using degrade proteins.
Verticals: HealthTech, Life Sciences, Oncology
Deal Date: 15-févr-2024
Deal Type: Early Stage VC
Deal Synopsis: The company raised $94 million of Series A venture funding in a deal led by Versant Ventures, MPM Capital and Decheng Capital on February 15, 2024.
Investors: Decheng Capital (Peter Colabuono), Eli Lilly (NYS: LLY) (Daniel Skovronsky), MPM Capital, Novartis (SWX: NOVN) (Vasant Narasimhan), Pappas Capital (Archie Griffin), RA Capital Management (Peter Kolchinsky), Sands Capital Ventures, Tavistock Group, Versant Ventures
Deal Size: $94.00
Freeline Therapeutics Holdings (NAS: FRLN)
Description: Operator of a gene therapy company intended to develop liver-directed gene therapies.
Verticals: Life Sciences
Deal Date: 12-févr-2024
Deal Type: PIPE
Deal Synopsis: The company (NAS:FRLN) is in talks to receive $50 million of development capital from Wellington Management through a private placement as of February 12, 2024.
Investors: Wellington Management
Deal Size: $50.00
Reduction in force (RIF)
February 15 - Aurinia Pharmaceuticals: After failing to attract a buyer, the biotech has resorted to trimming its workforce by 25% and cleaning out its R&D pipeline. Aurinia said the layoffs won't affect commercial or commercial-supporting roles. Story
February 13 - LianBio: The Chinese company has become the latest biotech to throw in the towel, with a prolonged dissolution expected closer to 2027. The biotech anticipates laying off 50 full-time employees—half of its staff—by the end of March, with “additional workforce reductions” due to occur during the year. Story
Disease of the week
Celiac disease, also known as coeliac disease, is a chronic autoimmune disorder that predominantly affects the small intestine. It occurs when individuals consume gluten, a protein found in wheat, barley, and rye. Upon ingesting gluten, the immune system launches an attack on the lining of the small intestine, resulting in inflammation and damage to the villi, which are tiny finger-like projections responsible for nutrient absorption.
Here are some key points about celiac disease:
Symptoms: Symptoms of celiac disease can vary widely and may include abdominal pain, bloating, diarrhea, constipation, weight loss, fatigue, anemia, joint pain, skin rash (dermatitis herpetiformis), and neurological symptoms such as headaches or peripheral neuropathy.
Diagnosis: Diagnosis usually involves a combination of blood tests and intestinal biopsy. Blood tests aim to detect specific antibodies produced in response to gluten, while an intestinal biopsy examines tissue samples from the small intestine to evaluate villi damage.
Prevalence: Celiac disease affects around 1% of the global population. It can manifest at any age but is commonly diagnosed in childhood or early adulthood.
Genetics: Celiac disease has a significant genetic component. Individuals with a family history of the condition are at higher risk. Certain genetic markers, particularly the HLA-DQ2 and HLA-DQ8 genes, are closely associated with the disease.
Treatment: The primary treatment for celiac disease involves strict adherence to a gluten-free diet. This means avoiding all sources of gluten, including wheat, barley, rye, and their derivatives. With a gluten-free diet, the intestinal lining typically heals, and symptoms improve. However, maintaining vigilance against gluten is crucial, as even small amounts can trigger a reaction.
Complications: Untreated celiac disease can lead to various complications, including malnutrition, osteoporosis, infertility, neurological disorders, and an increased risk of certain cancers such as lymphoma and small intestinal adenocarcinoma. Early diagnosis and adherence to a gluten-free diet are essential for preventing these complications.
Gluten Sensitivity vs. Celiac Disease: It's important to differentiate between celiac disease and non-celiac gluten sensitivity (NCGS). While celiac disease involves an autoimmune response and specific diagnostic criteria, NCGS manifests with similar symptoms but lacks autoimmune involvement or intestinal damage. The mechanisms and diagnostic criteria for NCGS are still being studied.
What I’ve read this week
*Click on the pic to read*