Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
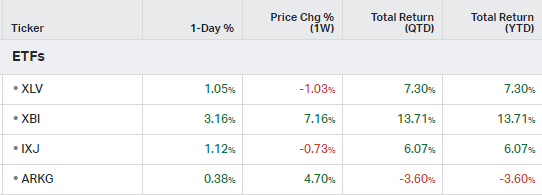
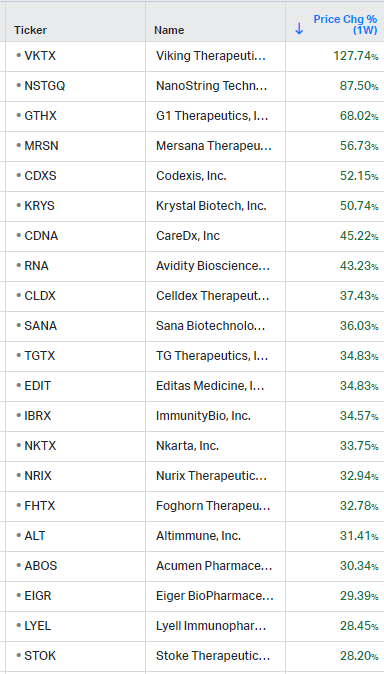
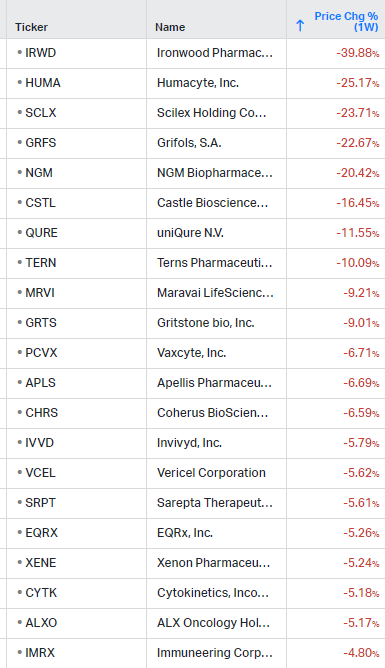
How the market performed this week
ETFs
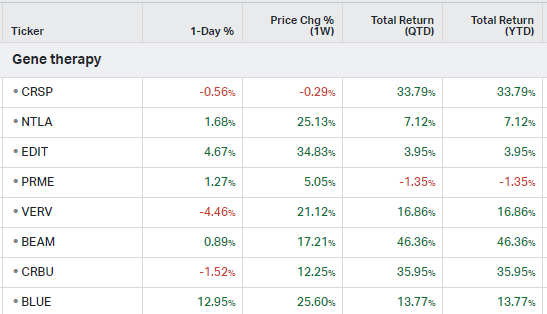
Gene Therapy
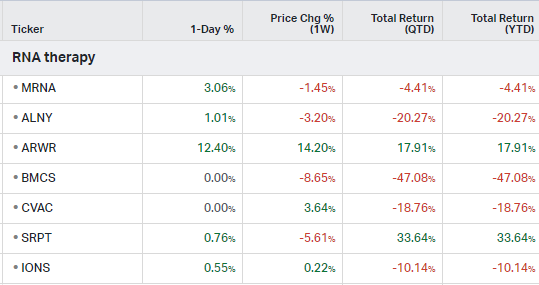
RNA Therapy
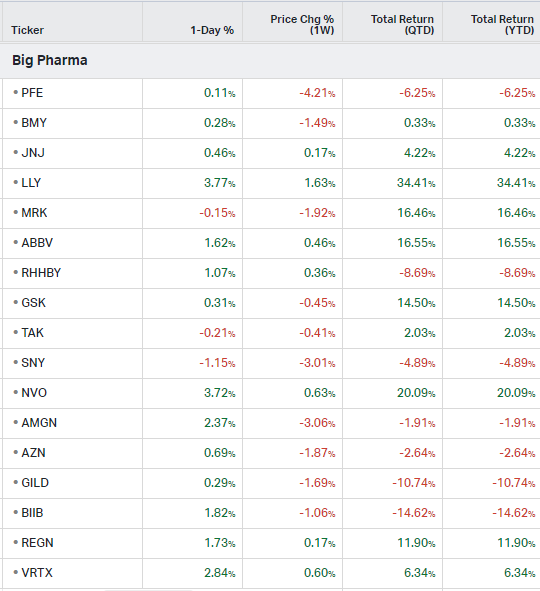
Big Pharma
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Arcutis Biotherapeutics, Inc. (ARQT)
Event Phase: NDA/BLA
Drug: Zoryve
Indication: Atopic Dermatitis (Eczema)
Lead Indication: No
Molecule: Small Molecule
Target: Phosphodiesterase 4 (PDE4)
LOA: 99%
Partner Companies: AstraZeneca PLC (AZN), Huadong Medicine Co., Ltd. (000963), Sato Pharmaceutical Co., Ltd.
Source Link: GlobeNewswire
Arcutis Biotherapeutics, Inc. (ARQT)
Event Phase: III
Drug: ZORYVE
Indication: Psoriasis
Lead Indication: No
Molecule: Small Molecule
Target: Phosphodiesterase 4 (PDE4)
LOA: 67%
Partner Companies: AstraZeneca PLC (AZN), Huadong Medicine Co., Ltd. (000963), Sato Pharmaceutical Co., Ltd.
Source Link: GlobeNewswire
Arcutis Biotherapeutics, Inc. (ARQT)
Event Phase: Approved
Drug: Zoryve
Indication: Psoriasis
Lead Indication: Yes
Molecule: Small Molecule
Target: Phosphodiesterase 4 (PDE4)
LOA: 100%
Partner Companies: AstraZeneca PLC (AZN), Huadong Medicine Co., Ltd. (000963), Sato Pharmaceutical Co., Ltd.
Source Link: GlobeNewswire
Amneal Pharmaceuticals, Inc. (AMRX)
Event Phase: NDA/BLA
Drug: IPX203
Indication: Parkinson's Disease (PD)
Lead Indication: Yes
Molecule: Small Molecule
Target: Aromatic L-amino acid decarboxylase (AADC), Dopamine Receptor - Unspecified
LOA: 97%
Partner Companies: Zambon Company S.p.A.
Source Link: BusinessWire
Teva Pharmaceutical Industries Ltd. (TEVA)
Event Phase: Approved
Drug: Austedo
Indication: Huntington's Disease
Lead Indication: Yes
Molecule: Small Molecule
Target: Vesicular monamine transporters (VMATs)
LOA: 100%
Partner Companies: Not specified
Source Link: BusinessWire
Bristol Myers Squibb Company (BMY)
Event Phase: III
Drug: RYZ101
Indication: Neuroendocrine Tumors (NET)
Lead Indication: No
Molecule: Small Molecule
Target: Unknown
LOA: 44%
Partner Companies: Not specified
Source Link: BusinessWire
Bristol Myers Squibb Company (BMY)
Event Phase: I
Drug: RYZ101
Indication: Small Cell Lung Cancer (SCLC)
Lead Indication: No
Molecule: Small Molecule
Target: Unknown
LOA: 5%
Partner Companies: Not specified
Source Link: BusinessWire
Bristol Myers Squibb Company (BMY)
Event Phase: Preclinical
Drug: RYZ801
Indication: Hepatocellular (Liver) Cancer (Including Secondary Metastases)
Lead Indication: Yes
Molecule: Peptide
Target: Glypican 3 (GPC3)
LOA: Not specified
Partner Companies: Ablaze Pharmaceuticals, PeptiDream Inc. (4587)
Source Link: BusinessWire
Bristol Myers Squibb Company (BMY)
Event Phase: Preclinical
Drug: CA9 Program (RayzeBio)
Indication: Renal Cell Cancer (RCC)
Lead Indication: No
Molecule: Small Molecule
Target: Carbonic Anhydrase
LOA: Not specified
Partner Companies: Not specified
Source Link: BusinessWire
FibroGen, Inc. (FGEN)
Event Phase: III
Drug: Roxadustat
Indication: Anemia Due to Chronic Kidney Disease, Dialysis-Dependent
Lead Indication: No
Molecule: Small Molecule
Target: Hypoxia-Inducible Factor-Prolyl Hydroxylase (HIF-PH)
LOA: 59%
Partner Companies: Astellas Pharma, Inc. (4503:JP), AstraZeneca PLC (AZN)
Source Link: GlobeNewswire
FibroGen, Inc. (FGEN)
Event Phase: III
Drug: Roxadustat
Indication: Anemia Due to Chronic Kidney Disease, Dialysis-Independent
Lead Indication: No
Molecule: Small Molecule
Target: Hypoxia-Inducible Factor-Prolyl Hydroxylase (HIF-PH)
LOA: 59%
Partner Companies: Astellas Pharma, Inc. (4503:JP), AstraZeneca PLC (AZN)
Source Link: GlobeNewswire
FibroGen, Inc. (FGEN)
Event Phase: III
Drug: Roxadustat
Indication: Cancer-Related Anemia
Lead Indication: No
Molecule: Small Molecule
Target: Hypoxia-Inducible Factor-Prolyl Hydroxylase (HIF-PH)
LOA: 63%
Partner Companies: Astellas Pharma, Inc. (4503:JP), AstraZeneca PLC (AZN)
Source Link: GlobeNewswire
Lixte Biotechnology Holdings, Inc. (LIXT)
Event Phase: II
Drug: LB-100
Indication: Ovarian Cancer
Lead Indication: No
Molecule: Small Molecule
Target: PP2A (Serine/threonine-specific protein phosphatase 2A)
LOA: 11%
Partner Companies: Not specified
Source Link: GlobeNewswire
NGM Biopharmaceuticals, Inc.
Event Phase: II
Drug: Aldafermin
Indication: Non-Alcoholic Steatohepatitis (NASH)
Lead Indication: No
Molecule: Protein
Target: Fibroblast Growth Factor Receptor (FGFR)
LOA: 9%
Partner Companies: Not specified
Source Link: GlobeNewswire
Merck & Co., Inc. (MRK)
Event Phase: II
Drug: MK-3655
Indication: Non-Alcoholic Steatohepatitis (NASH)
Lead Indication: No
Molecule: Monoclonal Antibody
Target: Fibroblast Growth Factor Receptor (FGFR)
LOA: 16%
Partner Companies: NGM Biopharmaceuticals, Inc. (NGM)
Source Link: GlobeNewswire
Clinical trials (LOA=likelihood of approval)
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: Approved
Drug: Zeposia
Indication: Multiple Sclerosis (MS)
Lead Indication: Yes
Molecule: Small Molecule
Target: Sphingosine 1-Phosphate Receptor (S1P-R)
LOA: 100%
Partner Companies: Not specified
Source Link: BusinessWire
Ironwood Pharmaceuticals, Inc.
Symbol: IRWD
Event Phase: III
Drug: Apraglutide
Indication: Short Bowel Syndrome (SBS)
Lead Indication: No
Molecule: Peptide
Target: GLP-2 Receptor
LOA: 52%
Partner Companies: Asahi Kasei Corporation (AHKSY), Ferring Pharmaceuticals, Swedish Orphan Biovitrum AB (SOBI)
Source Link: BusinessWire
Immunic Inc.
Symbol: IMUX
Event Phase: III
Drug: vidofludimus calcium
Indication: Multiple Sclerosis (MS)
Lead Indication: No
Molecule: Small Molecule
Target: Dihydroorotate Dehydrogenase (DHODH), DNA synthesis, Nuclear receptor related-1 protein (NURR1/NR4A2)
LOA: 52%
Partner Companies: 4SC AG (VSC)
Source Link: PRNewswire
Pfizer Inc.
Symbol: PFE
Event Phase: Approved
Drug: Abrysvo
Indication: Respiratory Syncytial Virus (RSV) Prevention
Lead Indication: Yes
Molecule: Vaccine
Target: Immune System, RSV, Respiratory Syncytial Virus
LOA: 100%
Partner Companies: Not specified
Source Link: BusinessWire
Cardiff Oncology, Inc.
Symbol: CRDF
Event Phase: II
Drug: Onvansertib
Indication: Colorectal Cancer (CRC)
Lead Indication: No
Molecule: Small Molecule
Target: Adenosine (including AMP, ADP, and ATP), Polo-like kinase 1 (Plk1)
LOA: 12%
Partner Companies: Nerviano Medical Sciences SRL
Source Link: GlobeNewswire
Clene, Inc.
Symbol: CLNN
Event Phase: II
Drug: CNM-Au8
Indication: Multiple Sclerosis (MS)
Lead Indication: No
Molecule: Small Molecule
Target: Myelin
LOA: 12%
Partner Companies: Not specified
Source Link: GlobeNewswire
Sonnet BioTherapeutics Holdings, Inc.
Symbol: SONN
Event Phase: II
Drug: SON-1010
Indication: Solid Tumors
Lead Indication: No
Molecule: Protein
Target: IL-12 (Interleukin-12) and IL-12 receptor, Immune System
LOA: 11%
Partner Companies: Not specified
Source Link: AccessWire
Imunon, Inc.
Symbol: IMNN
Event Phase: Preclinical
Drug: IMNN-101
Indication: COVID-19 Prevention
Lead Indication: Yes
Molecule: Vaccine
Target: IL-12 (Interleukin-12) and IL-12 receptor, SARS-CoV-2
LOA: Not specified
Partner Companies: Not specified
Source Link: GlobeNewswire
Silo Pharma, Inc.
Symbol: SILO
Event Phase: Preclinical
Drug: SPC-15
Indication: Post-Traumatic Stress Disorder (PTSD)
Lead Indication: No
Molecule: Not Specified
Target: Serotonin 5-HT4 receptor
LOA: Not specified
Partner Companies: Columbia University
Source Link: GlobeNewswire
Palatin Technologies, Inc.
Symbol: PTN
Event Phase: III
Drug: PL-9643
Indication: Dry Eye (Ophthalmology)
Lead Indication: Yes
Molecule: Peptide
Target: Melanocortin 1 Receptor (MC1R), Melanocortin-5 Receptor (MC5R)
LOA: 50%
Partner Companies: Not specified
Source Link: PR Newswire
Tonix Pharmaceuticals Holding Corp.
Symbol: TNXP
Event Phase: I
Drug: TNX-1500
Indication: Kidney Transplant Rejection
Lead Indication: No
Molecule: Monoclonal Antibody
Target: Cluster of Differentiation 154 (CD154) / CD40 Ligand (CD40L) / gp39
LOA: 11%
Partner Companies: Not specified
Source Link: GlobeNewswire
IGC Pharma, Inc.
Symbol: IGC
Event Phase: Preclinical
Drug: TGR-63
Indication: Alzheimer's Disease (AD)
Lead Indication: No
Molecule: Small Molecule
Target: Amyloid Beta/Amyloid Plaques
LOA: Not specified
Partner Companies: Not specified
Source Link: BusinessWire
Vivani Medical, Inc.
Symbol: VANI
Event Phase: Preclinical (NPM-115)
Drug: NPM-115
Indication: Obesity
Lead Indication: Yes
Molecule: Peptide
Target: GLP-1 Receptor
LOA: Not specified
Partner Companies: Not specified
Source Link: BusinessWire
Vivani Medical, Inc.
Symbol: VANI
Event Phase: IND (NPM-119)
Drug: NPM-119
Indication: Diabetes Mellitus, Type II
Lead Indication: Yes
Molecule: Peptide
Target: GLP-1 Receptor
LOA: Not specified
Partner Companies: Not specified
Source Link: BusinessWire
PureTech Health plc
Symbol: PRTC
Event Phase: I
Drug: LYT-200
Indication: Acute Myelogenous Leukemia (AML)
Lead Indication: No
Molecule: Monoclonal Antibody
Target: Galectin-9
LOA: 5%
Partner Companies: Not specified
Source Link: FDA
Nuvalent, Inc.
Symbol: NUVL
Event Phase: II
Drug: NVL-520
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: No
Molecule: Small Molecule
Target: ROS kinase
LOA: 11%
Partner Companies: Not specified
Source Link: PR Newswire
Viking Therapeutics, Inc.
Symbol: VKTX
Event Phase: II
Drug: VK2735
Indication: Obesity
Lead Indication: No
Molecule: Not specified
Target: GIP Receptor (GIPR)/Glucose-Dependent Insulinotropic Polypeptide Receptor, GLP-1 Receptor
LOA: 28%
Partner Companies: Not specified
Source Link: Not specified
Tonix Pharmaceuticals Holding Corp.
Symbol: TNXP
Event Phase: III
Drug: Tonmya
Indication: Fibromyalgia
Lead Indication: Yes
Molecule: Small Molecule
Target: Alpha 2 Adrenergic Receptor, Serotonin 5-HT2A receptor
LOA: 39%
Partner Companies: Not specified
Source Link: GlobeNewswire
GSK plc
Symbol: GSK
Event Phase: III
Drug: GSK2140944
Indication: Urinary Tract and Reproductive Tract Infections (Antibacterial)
Lead Indication: Yes
Molecule: Small Molecule
Target: Gram-Negative Bacteria, Topoisomerase II (DNA gyrase)
LOA: 71%
Partner Companies: Not specified
Source Link: GSK
Bayer AG
Symbol: BAYN
Event Phase: I
Drug: BAY 2927088
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: Yes
Molecule: Small Molecule
Target: EGFR (Epidermal Growth Factor Receptor), HER2/neu or ErbB-2
LOA: 5%
Partner Companies: Not specified
Source Link: BusinessWire
ARS Pharmaceuticals, Inc.
Symbol: SPRY
Event Phase: II
Drug: Neffy
Indication: Urticaria
Lead Indication: No
Molecule: Small Molecule
Target: Alpha Adrenergic Receptors, Beta Adrenergic Receptors
LOA: 18%
Partner Companies: Not specified
Source Link: GlobeNewswire
Sanofi
Symbol: SNY
Event Phase: Approved
Drug: Dupixent
Indication: Eosinophilic Esophagitis (EoE)
Lead Indication: Yes
Molecule: Monoclonal Antibody
Target: IL-13 (Interleukin-13), IL-4 Receptor (IL-4R)
LOA: 100%
Partner Companies: Regeneron Pharmaceuticals, Inc. (REGN)
Source Link: Journal of Allergy and Clinical Immunology
Orexo AB
Symbol: ORX
Event Phase: I
Drug: OX640
Indication: Allergy
Lead Indication: No
Molecule: Small Molecule
Target: Adrenergic Receptor - Unspecified
LOA: 10%
Partner Companies: Not specified
Source Link: Journal of Allergy and Clinical Immunology
OSE Immunotherapeutics
Symbol: OSE
Event Phase: Development Outside U.S.
Drug: OSE-279
Indication: Solid Tumors
Lead Indication: Yes
Molecule: Monoclonal Antibody
Target: IL-7 (Interleukin-7) and IL-7 receptor (IL-7R), Programmed death-1 receptor (PD-1)
LOA: Not specified
Partner Companies: Not specified
Source Link: BusinessWire
KalVista Pharmaceuticals, Inc.
Symbol: KALV
Event Phase: III
Drug: Sebetralstat
Indication: Hereditary Angioedema (HAE)
Lead Indication: Yes
Molecule: Small Molecule
Target: Kinin-Kallikrein System
LOA: 70%
Partner Companies: Not specified
Source Link: BusinessWire
Matinas BioPharma Holdings, Inc.
Symbol: MTNB
Event Phase: II
Drug: MAT-2203
Indication: Fungal Infections - Systemic
Lead Indication: No
Molecule: Small Molecule with Liposomal Delivery System
Target: Cell Membrane, Sterols
LOA: 23%
Partner Companies: Not specified
Source Link: GlobeNewswire
Tenaya Therapeutics, Inc.
Symbol: TNYA
Event Phase: Preclinical
Drug: TYA-018
Indication: Chronic Heart Failure - Preserved Ejection Fraction (Chronic HFpEF)
Lead Indication: No
Molecule: Small Molecule
Target: Histone Deacetylase (HDAC)
LOA: Not specified
Partner Companies: Not specified
Source Link: GlobeNewswire
Janux Therapeutics
Symbol: JANX
Event Phase: I
Drug: JANX007
Indication: Prostate Cancer
Lead Indication: Yes
Molecule: Cellular
Target: Prostate-specific Membrane Antigen (PSMA)/Folate hydrolase (FOLH1)
LOA: 5%
Partner Companies: Not specified
Source Link: BusinessWire
Janux Therapeutics
Symbol: JANX
Event Phase: I
Drug: JANX008
Indication: Solid Tumors
Lead Indication: No
Molecule: Cellular
Target: EGFR (Epidermal Growth Factor Receptor)
LOA: 5%
Partner Companies: Not specified
Source Link: BusinessWire
Tenaya Therapeutics, Inc.
Symbol: TNYA
Event Phase: I
Drug: TN-301
Indication: Chronic Heart Failure - Preserved Ejection Fraction (Chronic HFpEF)
Lead Indication: No
Molecule: Small Molecule
Target: Histone Deacetylase (HDAC)
LOA: 5%
Partner Companies: Not specified
Source Link: GlobeNewswire
Financing events
Absci (NAS: ABSI) - Public Investment 2nd Offering
Deal Date: 27-Feb-2024
Deal Type: Public Investment 2nd Offering
Deal Size: $75.15 million
Alamar Biosciences - Later Stage VC
Deal Date: 26-Feb-2024
Deal Type: Later Stage VC
Deal Size: $128 million
Applied Therapeutics (NAS: APLT) - PIPE
Deal Date: 27-Feb-2024
Deal Type: PIPE
Deal Size: $100 million
Avidity Biosciences (NAS: RNA) - PIPE
Deal Date: 29-Feb-2024
Deal Type: PIPE
Deal Size: $251.2 million
Crinetics Pharmaceuticals (NAS: CRNX) - PIPE
Deal Date: 28-Feb-2024
Deal Type: PIPE
Deal Size: $350 million
Curve Therapeutics - Early Stage VC
Deal Date: 27-Feb-2024
Deal Type: Early Stage VC
Deal Size: GBP 40.5 million
Denali Therapeutics (NAS: DNLI) - PIPE
Deal Date: 27-Feb-2024
Deal Type: PIPE
Deal Size: $500 million
Diagnos (TSX: ADK) - PIPE
Deal Date: 27-Feb-2024
Deal Type: PIPE
Deal Size: CAD 1.2 million
Egenesis - Later Stage VC
Deal Date: 27-Feb-2024
Deal Type: Later Stage VC
Deal Size: $25.42 million
EsgMax - Seed Round
Deal Date: 29-Feb-2024
Deal Type: Seed Round
Deal Size: EUR 700,000
Greenbrook TMS (PINX: GBNHF) - Public Investment 2nd Offering
Deal Date: 26-Feb-2024
Deal Type: Public Investment 2nd Offering
Deal Size: $1.20 million
Hemogenyx Pharmaceuticals (LON: HEMO) - PIPE
Deal Date: 29-Feb-2024
Deal Type: PIPE
Deal Size: GBP 3.33 million
Kenai Therapeutics - Early Stage VC
Deal Date: 29-Feb-2024
Deal Type: Early Stage VC
Deal Size: $82 million
Mainstay Medical - Later Stage VC
Deal Date: 26-Feb-2024
Deal Type: Later Stage VC
Deal Size: $125 million
Matter (Services (B2C Non-Financial)) - Later Stage VC
Deal Date: 26-Feb-2024
Deal Type: Later Stage VC
Deal Size: $26 million
Newtopia (TSX: NEWU) - PIPE
Deal Date: 29-Feb-2024
Deal Type: PIPE
Deal Size: $746,000
NRx Pharmaceuticals (NAS: NRXP) - Public Investment 2nd Offering
Deal Date: 28-Feb-2024
Deal Type: Public Investment 2nd Offering
Deal Size: $1.50 million
Orbis Medicines - Seed Round
Deal Date: 29-Feb-2024
Deal Type: Seed Round
Deal Size: EUR 26 million
Prolocor - Seed Round
Deal Date: 27-Feb-2024
Deal Type: Seed Round
Deal Size: $4.9 million
PulseSight Therapeutics - Seed Round
Deal Date: 28-Feb-2024
Deal Type: Seed Round
Pyxis Oncology (NAS: PYXS) - PIPE
Deal Date: 27-Feb-2024
Deal Type: PIPE
Deal Size: $50 million
RaaS (Decision/Risk Analysis) - Seed Round
Deal Date: 29-Feb-2024
Deal Type: Seed Round
Deal Size: $5 million
Revive Therapeutics (CNQ: RVV) - PIPE
Deal Date: 26-Feb-2024
Deal Type: PIPE
Deal Size: CAD 2.1 million
Sonichem - Later Stage VC
Deal Date: 29-Feb-2024
Deal Type: Later Stage VC
Deal Size: GBP 1.2 million
Sonichem - Series A VC
Deal Date: 29-Feb-2024
Deal Type: Series A VC
vTv Therapeutics (NAS: VTVT) - PIPE
Deal Date: 28-Feb-2024
Deal Type: PIPE
Deal Size: $51 million
Yuva Biosciences - Seed Round
Deal Date: 26-Feb-2024
Deal Type: Seed Round
Deal Size: $7.5 million
Reduction in force (RIF)
February 29 - Kineta: After a strategic review, the small oncology biotech is conducting a corporate restructuring and reducing its workforce by 64%, or seven positions, including CEO Shawn Iadonato, Ph.D. Story
February 28 - Johnson & Johnson: The pharma is closing a nearly 200,000 square-foot R&D outpost in Brisbane, California, less than 18 months after it opened. In conjunction with the closure, the company has implemented 55 permanent layoffs that will go into effect by April 26. Story
February 28 - ObsEva: With its money and options dwindling, ObsEva has finally decided to wind down operations and lay off all employees. Story
February 28 - Nicox: The ophthalmology biotech has agreed to an amendment to the repayment of a $14.5 million debt with Kreos Capital, which will extend the company’s cash runway to November. But, to secure this deal, Nicox had to agree to “reduce its operations in France and Italy” as well as cut operating costs and restructure the company. Story
February 28 - Longeveron: The regenerative medicine biotech is discontinuing a phase 2 trial of its lead candidate Lomecel-B in aging-related frailty in Japan, a move that will result in “related staff reductions” that the company listed as part of “cost-saving measures.” Story
February 26 - Denali Therapeutics: The biotech is reorganizing, laying off an undisclosed number of workers and sending some to a new spin-out. A company spokesperson said the workforce reduction was “considerably less than 10%” of the total team. Story
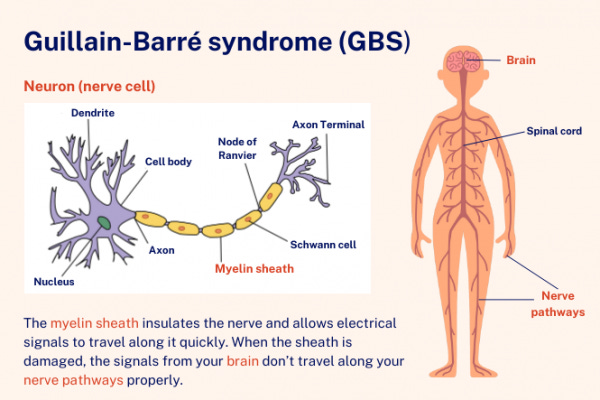
Disease of the week
Guillain-Barré syndrome (GBS) is a rare but serious autoimmune disorder affecting the peripheral nervous system, characterized by rapid onset muscle weakness, occasionally progressing to paralysis. Here's a detailed overview:
Causes:
GBS occurs when the body's immune system mistakenly attacks the peripheral nerves, damaging the myelin sheath (the protective covering of nerves) or the nerves themselves.
The exact cause is often unknown, but it is frequently preceded by an infection, typically a bacterial or viral infection like Campylobacter jejuni, Epstein-Barr virus, cytomegalovirus (CMV), or Zika virus.
It can also sometimes occur after surgery or vaccination.
Symptoms:
GBS often starts with tingling or weakness in the legs, which can quickly spread to the arms and upper body.
Symptoms typically progress rapidly, reaching their peak within a few weeks.
Common symptoms include muscle weakness or paralysis, difficulty with eye movements, difficulty speaking, difficulty swallowing, and sometimes even difficulty with breathing.
Types:
The most common form is acute inflammatory demyelinating polyneuropathy (AIDP), where the immune system attacks the myelin sheath of nerves.
Another form is acute motor axonal neuropathy (AMAN), where the immune system attacks the nerve axons themselves.
There are other less common variants as well, including acute motor-sensory axonal neuropathy (AMSAN) and Miller Fisher syndrome.
Diagnosis:
Diagnosis is based on the patient's symptoms, physical examination, and sometimes electrophysiological studies such as nerve conduction studies and electromyography (EMG).
Lumbar puncture (spinal tap) may also be done to analyze cerebrospinal fluid for signs of inflammation.
Treatment:
There is no cure for GBS, but treatments can help speed up recovery and reduce the severity of symptoms.
The mainstay of treatment is intravenous immunoglobulin (IVIG) or plasmapheresis (plasma exchange), which help to reduce the immune system's attack on the nerves.
Supportive care such as physical therapy, occupational therapy, and respiratory support may be necessary, especially if there is paralysis or difficulty breathing.
Prognosis:
Most people with Guillain-Barré syndrome eventually recover, although the recovery process can be slow and may take weeks to months.
Some individuals may experience residual weakness or other long-term complications.
In severe cases, complications such as respiratory failure or autonomic dysfunction can be life-threatening.
What I’ve read this week
*Click on the pic to read*