Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
How the market performed this week
ETFs
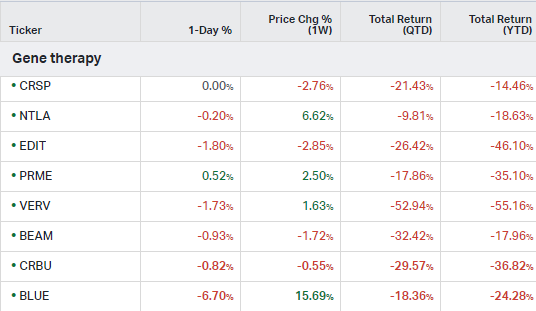
Gene Therapy
RNA Therapy
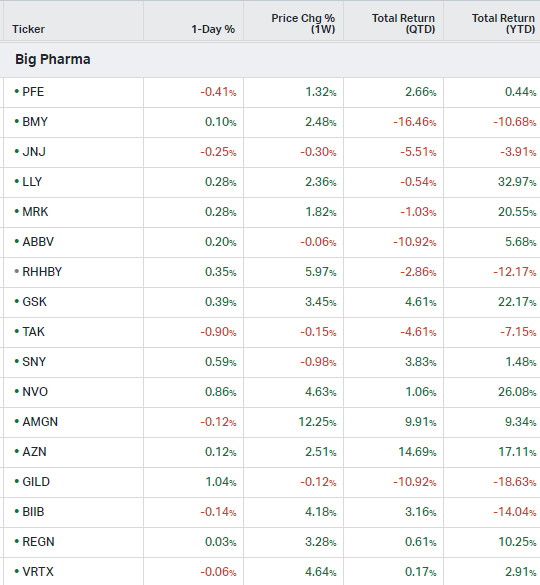
Big Pharma
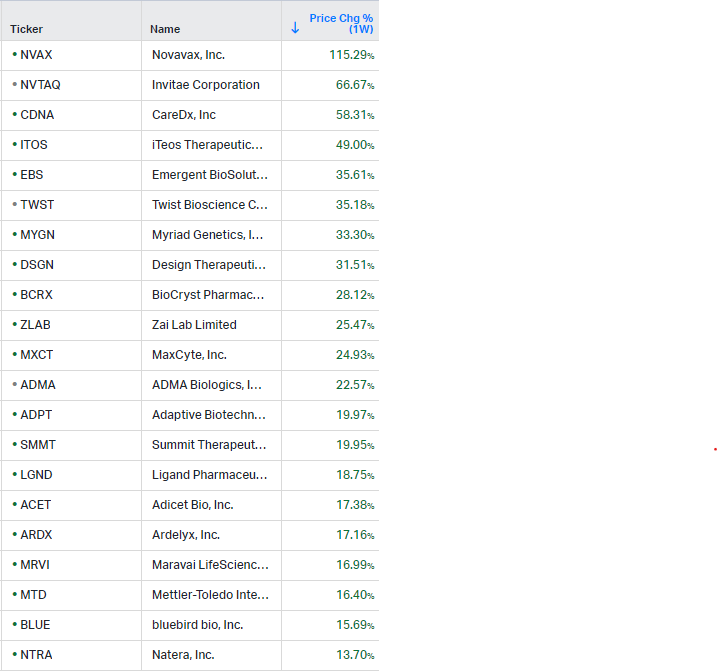
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Shionogi & Co. Ltd.
Symbol: 4507
Event Type: Partnership - Licensing Deal
Current Phase: I
Drug: MZE001
Disease Group: Metabolic
Indication: Pompe Disease
Target: Glycogen Synthase 1 (GYS1)
LOA: 15%
Source Link: BusinessWire
Novavax, Inc.
Symbol: NVAX
Event Type: Partnership - Licensing Deal
Current Phase: BLA
Drug: Nuvaxovid
Disease Group: Infectious Disease
Indication: COVID-19 Prevention
Target: Immune System, Influenza Virus, SARS-CoV-2
LOA: 99%
Source Link: PR Newswire
NovaBay Pharmaceuticals, Inc.
Symbol: NBY
Event Type: Partnership - Licensing Deal (Emerging Markets)
Current Phase: Approved
Drug: APP13007
Disease Group: Ophthalmology
Indication: Ocular Pain and/or Inflammation (Ophthalmology)
Target: Glucocorticoid Receptor (GR)
LOA: 100%
Source Link: PR Newswire
Century Therapeutics, Inc.
Symbol: IPSC
Event Type: Partnership - Acquisition Closed
Current Phases: I, Preclinical
Drugs: GDT-002, GDT201, TEG Program (Gadeta)
Disease Group: Oncology
Indications: Multiple Myeloma, Solid Tumors
Targets: Immune System, Stem Cells/Other Cell Therapies, T-Cell Receptor (TCR), Autologous Chimeric Antigen Receptor T-cells (CAR-T)
LOA: 5% (GDT-002)
Source Link: GlobeNewswire
Coherus BioSciences, Inc.
Symbol: CHRS
Event Type: Partnership - Announcement
Current Phase: Preclinical
Drug: Loqtorzi
Disease Group: Oncology
Indication: Ovarian Cancer
Target: Immune System, Programmed death-1 receptor (PD-1)
Source Link: GlobeNewswire
Invion Limited
Symbol: IVX
Event Type: Partnership - Announcement
Current Phase: Development Outside U.S.
Drug: INV043
Disease Group: Oncology
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Target: Immune System
Source Link: Investors Inviongroup
Santen Pharmaceutical Co., Ltd.
Symbol: 4536
Event Type: Partnership - Distribution Agreement
Current Phase: Approved in other than U.S./E.U.
Drug: Alesion
Disease Group: Ophthalmology
Indication: Allergic Conjunctivitis (Ophthalmology)
Target: Histamine H1 Receptor (HRH1)
Source Link: Santen
GSK plc
Symbol: GSK
Event Type: Partnership - Announcement
Current Phase: Preclinical
Drug: BVL-GSK098
Disease Group: Infectious Disease
Indication: Tuberculosis
Target: Tuberculosis
Source Link: GlobeNewswire
Clinical trials (LOA=likelihood of approval)
Aurinia Pharmaceuticals Inc.
Symbol: AUPH
Event Phase: Approved
Trial Name: Phase III - AURORA 1, Phase III - AURORA 2
Drug: Lupkynis
Disease Group: Autoimmune/immunology
Indication: Lupus Nephritis
Target: Calcineurin phosphatase
LOA: 100%
Partner Companies: 3SBio Inc. (1530), CSL Vifor, Getinge AB (GNGBY), Lux Biosciences, Inc., Otsuka Holdings Co., Ltd. (4578)
Source Link: BusinessWire
Fate Therapeutics, Inc.
Symbol: FATE
Event Phase: I
Trial Name: Phase I - Dose-Finding (B-Cell Malignancies)
Drug: FT819
Disease Group: Oncology
Indication: Hematologic Cancer
Target: Allogeneic Chimeric Antigen Receptor T-cells (CAR-T), Autologous Chimeric Antigen Receptor T-cells (CAR-T), Cluster of Differentiation 19 (CD19), Immune System, Stem Cells/Other Cell Therapies
LOA: 5%
Partner Companies: Memorial Sloan Kettering Cancer Center
Source Link: GlobeNewswire
Kodiak Sciences Inc.
Symbol: KOD
Event Phase: I
Trial Name: Phase I - First-in-Human Study
Drug: KSI-501
Disease Group: Ophthalmology
Indication: Other Ophthalmological Indications (Ophthalmology)
Target: IL-6 (Interleukin-6), VEGF (Vascular endothelial growth factor)
LOA: 21%
Source Link: ARVO24
Editas Medicine
Symbol: EDIT
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: AsCas12a
Disease Group: Ophthalmology
Indication: Glaucoma / Ocular Hypertension (Ophthalmology)
Target: Unknown
Source Link: Editas Medicine
Ocular Therapeutix, Inc.
Symbol: OCUL
Event Phase: I
Trial Name: Preclinical Studies
Drug: AXPAXLI
Disease Group: Ophthalmology
Indication: Diabetic Retinopathy (Ophthalmology)
Target: Tyrosine Kinases
LOA: 21%
Source Link: ARVO24
Celularity, Inc.
Symbol: CELU
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: CYNK-201
Disease Group: Oncology
Indication: Cancer
Target: Unknown
Source Link: ASGCT
Fate Therapeutics, Inc.
Symbol: FATE
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: FT-522
Disease Group: Autoimmune/immunology
Indication: Systemic Lupus Erythematosus (SLE)
Target: Cluster of Differentiation 19 (CD19), Natural Killer Cells (NK Cells)
Source Link: GlobeNewswire
Fate Therapeutics, Inc.
Symbol: FATE
Event Phase: I
Trial Name: Phase I - (w/Rituximab)
Drug: FT-522
Disease Group: Oncology
Indication: Non-Hodgkin's Lymphoma (NHL)
Target: Cluster of Differentiation 19 (CD19), Natural Killer Cells (NK Cells)
LOA: 5%
Source Link: GlobeNewswire
Regeneron Pharmaceuticals, Inc.
Symbol: REGN
Event Phase: Approved
Trial Name: Phase II/III - PHOTON
Drug: Eylea
Disease Group: Ophthalmology
Indication: Diabetic Macular Edema
Target: Placental growth factor (PlGF), VEGF (Vascular endothelial growth factor)
LOA: 100%
Partner Companies: Bayer AG (BAYN), DRI Capital Inc., Roche Holding AG (RHHBY), Santen Pharmaceutical Co., Ltd. (4536)
Source Link: ARVO24
Regeneron Pharmaceuticals, Inc.
Symbol: REGN
Event Phase: II
Trial Name: Phase I/II - CHORD
Drug: DB-OTO
Disease Group: ENT/Dental
Indication: Otoferlin Gene-Mediated Hearing Loss
Target: Otoferlin
LOA: 24%
Source Link: GlobeNewswire
PDS Biotechnology Corporation
Symbol: PDSB
Event Phase: II
Trial Name: Phase II - VERSATILE-002 (US/UK)
Drug: Versamune-HPV
Disease Group: Oncology
Indication: Head and Neck Cancer
Target: Human Papillomavirus (HPV), Immune System
LOA: 13%
Source Link: GlobeNewswire
ProQR Therapeutics N.V.
Symbol: PRQR
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: AX-0810
Disease Group: Gastroenterology (Non Inflammatory Bowel Disease)
Indication: Liver Failure / Cirrhosis
Target: Unknown
Source Link: ASGCT
Cytokinetics, Inc.
Symbol: CYTK
Event Phase: I
Trial Name: Phase I - SAD/MAD
Drug: CK-4021586
Disease Group: Cardiovascular
Indication: Chronic Heart Failure - Preserved Ejection Fraction (Chronic HFpEF)
Target: Myosin
LOA: 5%
Source Link: GlobeNewswire
Amgen, Inc.
Symbol: AMGN
Event Phase: I
Trial Name: Phase Ia - First-in-Human
Drug: CX-904
Disease Group: Oncology
Indication: Cancer
Target: Cluster of Differentiation 3 (CD3), EGFR (Epidermal Growth Factor Receptor)
LOA: 5%
Partner Companies: CytomX Therapeutics, Inc. (CTMX)
Source Link: GlobeNewswire
Prime Medicine, Inc.
Symbol: PRME
Event Phase: IND
Trial Name: Preclinical Studies
Drug: PM-359
Disease Group: Autoimmune/immunology
Indication: Chronic Granulomatous Disease
Target: Allogeneic Chimeric Antigen Receptor T-cells (CAR-T), Immune System
Source Link: GlobeNewswire
Carisma Therapeutics Inc.
Symbol: CARM
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: Engineered Macrophage Cell Therapy (Carisma)
Disease Group: Autoimmune/immunology and Respiratory
Indication: Hepatic Fibrosis and Pulmonary Fibrosis
Target: Unknown
Source Link: PRNewswire
NanoViricides, Inc.
Symbol: NNVC
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: NV-CoV-2
Disease Group: Infectious Disease
Indication: Smallpox
Target: SARS-CoV-2
Partner Companies: Karveer Meditech Pvt. Ltd.
Source Link: AccessWire
Eli Lilly and Company
Symbol: LLY
Event Phase: I
Trial Name: Phase III - DREAMS-2 (China)
Drug: Mazdutide
Disease Group: Endocrine
Indication: Diabetes Mellitus, Type II
Target: GLP-1 Receptor, Glucagon Receptor
LOA: 7%
Partner Companies: Innovent Biologics, Inc. (1801)
Source Link: PR Newswire
NanoViricides, Inc.
Symbol: NNVC
Event Phase: Preclinical
Drug: NV-CoV-2
Disease Group: Infectious Disease
Indication: Monkeypox (Mpox)
Target: SARS-CoV-2
Partner Companies: Karveer Meditech Pvt. Ltd.
Source Link: AccessWire
Moleculin Biotech, Inc.
Symbol: MBRX
Event Phase: II
Trial Name: Phase I/II - MB-106
Drug: Annamycin
Disease Group: Oncology
Indication: Acute Myelogenous Leukemia (AML)
Target: Topoisomerase II (DNA gyrase)
LOA: 11%
Partner Companies: University of Texas MD Anderson Cancer Center
Source Link: PR Newswire
Barinthus Biotherapeutics plc
Symbol: BRNS
Event Phase: Suspended
Trial Name: Phase IIb - FLU010 (Belgium)
Drug: VTP-100
Disease Group: Infectious Disease
Indication: Influenza (including vaccines)
Target: Immune System, Influenza Virus
Source Link: The Lancet
Kodiak Sciences Inc.
Symbol: KOD
Event Phase: III
Trial Name: Phase III - GLOW
Drug: KSI-301
Disease Group: Ophthalmology
Indication: Diabetic Retinopathy
Target: VEGF (Vascular endothelial growth factor)
LOA: 54%
Source Link: PR Newswire
Eledon Pharmaceuticals, Inc.
Symbol: ELDN
Event Phase: II
Trial Name: Phase Ib/II - (Canada/UK)
Drug: Tegoprubart
Disease Group: Autoimmune/immunology
Indication: Kidney Transplant Rejection
Target: Cluster of Differentiation 154 (CD154) / CD40 Ligand (CD40L) / gp39, Fc receptors, Lymphocytes
LOA: 19%
Source Link: GlobeNewswire
Annexon, Inc.
Symbol: ANNX
Event Phase: II
Trial Names: Phase II - ARCHER, Preclinical Studies
Drug: ANX-007
Disease Group: Ophthalmology
Indication: Dry Age-Related Macular Degeneration (Dry AMD)/Geographic Atrophy
Target: Complement Component 1q (C1q), Complement Pathway
LOA: 23%
Source Link: GlobeNewswire
Financing events
Aardvark Therapeutics
Description: Develops small molecule therapeutics for obesity and rare genetic metabolic diseases.
Verticals: Life Sciences
Deal Date: May 9, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $85 million in Series C funding led by Decheng Capital to complete clinical trials and regulatory approval for ARD-101 and other pipeline programs.
Investors: Decheng Capital, Cantor Fitzgerald, Cormorant Asset Management, FPWR, Laurion Capital Management, LG Technology Ventures, Prader-Willi Syndrome Association, SilverArc Capital Management, Surveyor Capital, SymBiosis Capital Management, Tetragon Financial Group, Vickers Venture Partners, Walleye Capital
Deal Size: $85 million
ADC Therapeutics (NYS: ADCT)
Description: Commercial-stage oncology-focused biotech developing antibody-drug conjugates.
Verticals: Life Sciences, Oncology
Deal Date: May 7, 2024
Deal Type: Public Investment 2nd Offering
Deal Synopsis: Conducted a second public offering, raising $65.718 million by selling 13,411,912 shares at $4.9 each.
Deal Size: $65.72 million
Alzamend Neuro (NAS: ALZN)
Description: Early clinical-stage biopharmaceutical company focused on neurodegenerative diseases and psychiatric disorders.
Verticals: Life Sciences
Deal Date: May 9, 2024
Deal Type: PIPE
Deal Synopsis: In talks to receive $25 million through a private placement for clinical trials and working capital.
Deal Size: $25 million
Apollomics (NAS: APLM)
Description: Clinical-stage biopharmaceutical company developing oncology therapies.
Verticals: Life Sciences, Oncology
Deal Date: May 8, 2024
Deal Type: PIPE
Deal Synopsis: Aiming to receive $5.75 million in development capital through a private placement.
Deal Size: $5.75 million
Atacama Therapeutics
Description: Develops dermatology-focused products, specifically targeting hyperhidrosis.
Verticals: HealthTech, Life Sciences, LOHAS & Wellness
Deal Date: May 6, 2024
Deal Type: Seed Round
Deal Synopsis: Raised $830,000 in seed funding.
Deal Size: $0.83 million
Attovia
Description: Develops a biotherapeutics pipeline for immune-mediated disease and oncology using a nanobody platform.
Verticals: Life Sciences, Oncology
Deal Date: May 9, 2024
Deal Type: Early Stage VC
Deal Synopsis: Raised $105 million in Series B funding led by The Goldman Sachs Group to advance lead programs and develop the ATTOBODY™ platform.
Investors: The Goldman Sachs Group, Cormorant Asset Management, EcoR1 Capital, Frazier Healthcare Partners, Illumina Ventures, Logos Capital, Marshall Wace, Nextech Ventures, Redmile Group, venBio
Deal Size: $105 million
BioVersys
Description: Focuses on developing treatments against bacterial infections.
Verticals: Life Sciences
Deal Date: May 7, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised CHF 45.71 million in Series C funding for Phase II trials of BV100 and BVL-GSK098 and preclinical development of BV200.
Investors: GSK, Clinical Research Ventures, AMR Action Fund
Deal Size: CHF 50.18 million (approx. $50.18 million)
Bluejay Therapeutics
Description: Develops treatments for serious viral and liver diseases.
Verticals: Life Sciences, LOHAS & Wellness
Deal Date: May 9, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $182 million in Series C funding to progress candidates for chronic hepatitis B treatment.
Investors: Frazier Lifesciences Acquisition, Arkin Bio Ventures, HBM Healthcare Investments, Novo Holdings, Octagon Capital Advisors, RA Capital Management, RiverVest Venture Partners, T. Rowe Price, Unicorn Capital Partners, Wellington Management
Deal Size: $182 million
Reduction in force (RIF)
May 9 - Ginkgo Bioworks: After “disappointing” first-quarter revenues, Ginkgo is accelerating plans to break even by the end of 2026, and is now setting its sights on reducing operating costs by $200 million by the middle of next year. A key part of this program will be a “reduction in labor expenses of at least 25%, across both G&A and R&D functions,” the company explained in an earnings report. While this will include a “reduction in force,” the company did not put a figure on how many employees would be impacted. Release
May 9 - Pfizer: The pharma, which completed its $43 billion buyout of ADC specialist Seagen in December, has made the “difficult decision” to propose another round of staff reductions and role changes at the newly acquired company, a Pfizer spokesperson said. This time, the proposal will hit Seagen’s European headquarters in Switzerland, where 74 positions could be up for the chop. More jobs could be on the line if Pfizer isn’t able to transfer an additional 21 employees into new roles. Story
May 8 - Marinus: As part of a wider cost-cutting plan, the Pennsylvania is jettisoning a fifth of its workforce. The company also mentioned “additional cost reductions across both R&D and general and administrative functions." Story
May 7 - Kenvue: The company's board of directors approved a plan to cut 4% of its global workforce as a “transition service agreement” with J&J winds down. The company employed about 23,000 employees at the end of last year, so the layoff initiative could affect some 920 workers. Story
Disease of the week
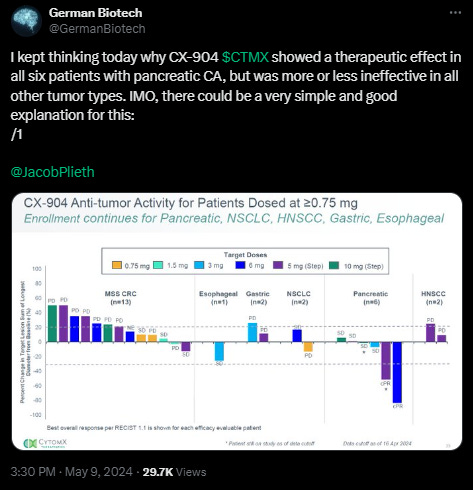
What I’ve read this week
*Click on the pic to read*