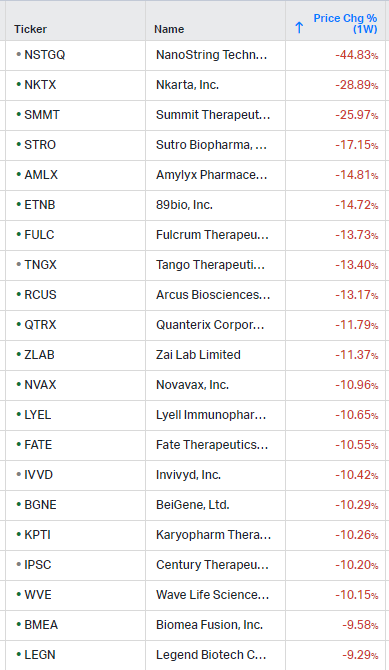
Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
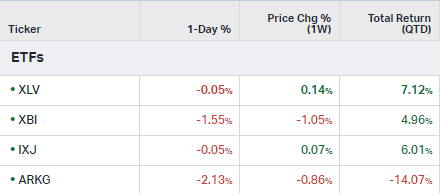
How the market performed this week
ETFs
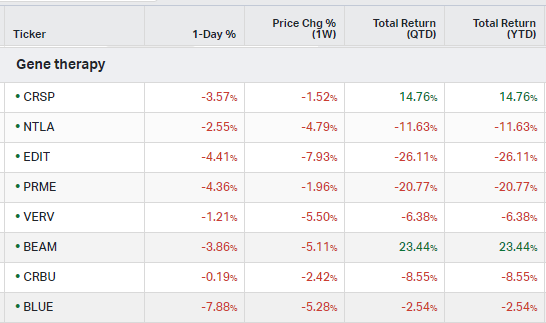
Gene Therapy
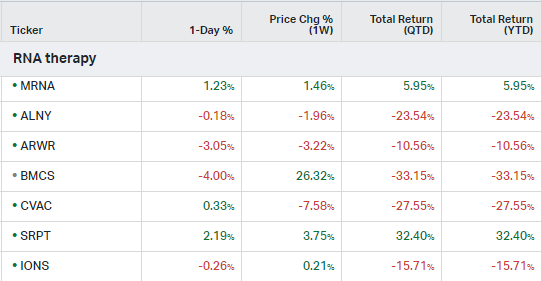
RNA Therapy
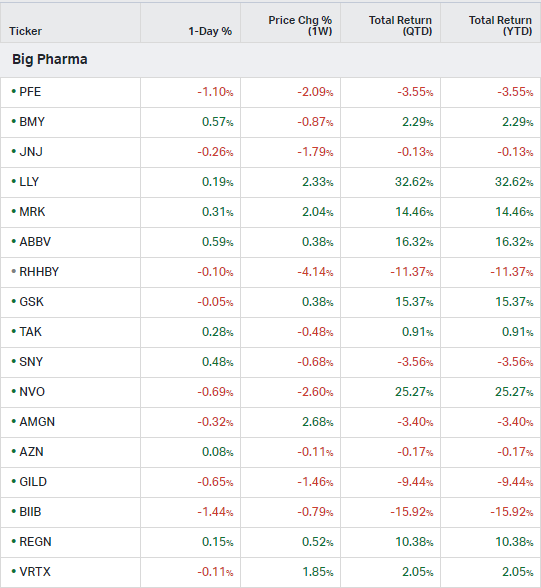
Big Pharma
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Sosei Heptares
Symbol: 4565
Event Phase: Development Outside U.S.
Drug: HTL0027477
Disease Group: Autoimmune/immunology
Indication: Ulcerative Colitis (UC)
Target: Unknown
LOA: Not specified
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: II
Drug: 225Ac-PSMA I&T
Disease Group: Oncology
Indication: Prostate Cancer
Target: Prostate-specific Membrane Antigen (PSMA)/Folate hydrolase (FOLH1)
LOA: 11%
Source Link: Link
Nascent Biotech, Inc.
Symbol: NBIO
Event Phase: II
Drug: Pritumumab
Disease Group: Oncology
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Target: Vimentin
LOA: 11%
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: II
Drug: FPI-1966
Disease Group: Oncology
Indication: Bladder Cancer
Target: Fibroblast Growth Factor Receptor (FGFR)
LOA: 11%
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: II
Drug: FPI-1966
Disease Group: Oncology
Indication: Head and Neck Cancer
Target: Fibroblast Growth Factor Receptor (FGFR)
LOA: 11%
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: I
Drug: FPI-2058
Disease Group: Oncology
Indication: Solid Tumors
Target: NT1 (Neurotensin receptor type 1)
LOA: 5%
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: I
Drug: FPI-1434
Disease Group: Oncology
Indication: Solid Tumors
Target: IGF-1R (Insulin-like Growth Factor-1 Receptor)
LOA: 5%
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: Preclinical
Drug: FPI-2059
Disease Group: Oncology
Indication: Colorectal Cancer (CRC)
Target: NT1 (Neurotensin receptor type 1)
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: I
Drug: FPI-2059
Disease Group: Oncology
Indication: Solid Tumors
Target: NT1 (Neurotensin receptor type 1)
LOA: 5%
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: IND
Drug: FPI-2068
Disease Group: Oncology
Indication: Cancer
Target: Unknown
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: Preclinical
Drug: FPI-1848
Disease Group: Oncology
Indication: Sarcoma - Unspecified
Target: Cluster of Differentiation 248 (CD248)/Endosialin
Source Link: Link
Fusion Pharmaceuticals Inc.
Symbol: FUSN
Event Phase: Preclinical
Drug: FPI-1792
Disease Group: Oncology
Indication: Colorectal Cancer (CRC)
Target: IGF-1R (Insulin-like Growth Factor-1 Receptor)
Source Link: Link
BiomX Inc.
Symbol: PHGE
Event Phase: II
Drug: BX211
Disease Group: Infectious Disease
Indication: Bone and Joint Infections (Antibacterial)
Target: Bacteria-miscellaneous
LOA: 23%
Source Link: Link
BiomX Inc.
Symbol: PHGE
Event Phase: II
Drug: BX211
Disease Group: Infectious Disease
Indication: COVID-19 Treatment
Target: Bacteria-miscellaneous
LOA: 23%
Source Link: Link
BiomX Inc.
Symbol: PHGE
Event Phase: II
Drug: BX211
Disease Group: Respiratory
Indication: Cystic Fibrosis (CF)
Target: Bacteria-miscellaneous
LOA: 13%
Source Link: Link
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: NDA/BLA
Drug: KarXT
Disease Group: Psychiatry
Indication: Schizophrenia
Target: Muscarinic acetylcholine receptor, Muscarinic M1 receptor
LOA: 99%
Source Link: Link
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: III
Drug: KarXT
Disease Group: Psychiatry
Indication: Neuropsychiatric Symptoms in Alzheimer’s Disease
Target: Muscarinic acetylcholine receptor, Muscarinic M1 receptor
LOA: 51%
Source Link: Link
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: I
Drug: KAR-2618
Disease Group: Psychiatry
Indication: Major Depressive Disorder (MDD)
Target: TRPC5, transient receptor potential canonical 5
LOA: 7%
Source Link: Link
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: Preclinical
Drug: KAR-201
Disease Group: Not Specified
Indication: Undisclosed
Target: Muscarinic acetylcholine receptor
Source Link: Link
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: Preclinical
Drug: KAR-301
Disease Group: Not Specified
Indication: Undisclosed
Target: Muscarinic acetylcholine receptor
Source Link: Link
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: Preclinical
Drug: KAR-401
Disease Group: Not Specified
Indication: Undisclosed
Target: Muscarinic acetylcholine receptor
Source Link: Link
Bristol Myers Squibb Company
Symbol: BMY
Event Phase: Preclinical
Drug: KAR-501
Disease Group: Not Specified
Indication: Undisclosed
Target: Unknown
Source Link: Link
CytomX Therapeutics, Inc.
Symbol: CTMX
Event Phase: Preclinical
Drug: Probody T-Cell Therapy (CytomX)
Disease Group: Oncology
Indication: Cancer
Target: Cluster of Differentiation 3 (CD3), Immune System
Source Link: Link
Clinical trials (LOA=likelihood of approval)
Phio Pharmaceuticals Corp.
Symbol: PHIO
Event Type: Trial Data - Preclinical Results
Event Phase: Preclinical
Drug: PH-905
Disease Group: Oncology
Indication: Hematologic Cancer
Target: CBL-B
Source Link: Link
Alpine Immune Sciences Inc.
Symbol: ALPN
Event Type: Trial Data - Preclinical Results
Event Phase: I
Drug: ALPN-303
Disease Group: Autoimmune/immunology
Indication: Systemic Lupus Erythematosus (SLE)
Target: APRIL, B-cell activating factor (BAFF)/B-lymphocyte stimulator (BLyS)
Source Link: Link
Roche Holding AG
Symbol: RHHBY
Event Type: Trial Data - Top-Line Results
Event Phase: III
Drug: Enspryng
Disease Group: Autoimmune/immunology
Indication: Myasthenia Gravis (MG)
Target: IL-6 Receptor (IL-6R)
Source Link: Link
AstraZeneca PLC
Symbol: AZN
Event Type: Trial Data - Updated Results
Event Phase: Approved
Drug: Tagrisso
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: EGFR (Epidermal Growth Factor Receptor)
Source Link: Link
Merck & Co., Inc.
Symbol: MRK
Event Type: Trial Data - Top-Line Results
Event Phase: Approved
Drug: Keytruda
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Immune System, Programmed death-1 receptor (PD-1)
Source Link: Link
Johnson & Johnson
Symbol: JNJ
Event Type: Trial Data - Updated Results
Event Phase: Approved
Drug: Rybrevant
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: EGFR (Epidermal Growth Factor Receptor), Hepatocyte growth factor receptor (c-Met, HGFR)
Source Link: Link
Johnson & Johnson
Symbol: JNJ
Event Type: Trial Data - Updated Results
Event Phase: Approved
Drug: Rybrevant
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: EGFR (Epidermal Growth Factor Receptor), Hepatocyte growth factor receptor (c-Met, HGFR)
Source Link: Link
Johnson & Johnson
Symbol: JNJ
Event Type: Trial Data - Updated Results
Event Phase: III
Drug: Amivantamab SQ
Disease Group: Oncology
Indication: Solid Tumors
Target: EGFR (Epidermal Growth Factor Receptor), Tyrosine Kinases
Source Link: Link
Johnson & Johnson
Symbol: JNJ
Event Type: Trial Data - Updated Results
Event Phase: Approved
Drug: Rybrevant
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: EGFR (Epidermal Growth Factor Receptor), Hepatocyte growth factor receptor (c-Met, HGFR)
Source Link: Link
Bristol Myers Squibb Company
Symbol: BMY
Event Type: Trial Data
Event Phase: Approved
Drug: Opdivo
Disease Group: Oncology
Indication: Hepatocellular (Liver) Cancer (HCC) (Including Secondary Metastases)
Target: Immune System, Programmed death-1 receptor (PD-1)
Source Link: Link
Precision BioSciences, Inc.
Symbol: DTIL
Event Type: Trial Data - Preclinical Results
Event Phase: Preclinical
Drug: PBGENE-PMM
Disease Group: Metabolic
Indication: Primary Mitochondrial Myopathies (PMM)
Target: Mitochondria
Source Link: Link
Kiromic BioPharma, Inc.
Symbol: KRBP
Event Type: Trial Data - Updated Results
Event Phase: I
Drug: Deltacel
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Unknown
Source Link: Link
Hoth Therapeutics, Inc.
Symbol: HOTH
Event Type: Trial Data - Preclinical Results
Event Phase: Preclinical
Drug: HT-ALZ
Disease Group: Neurology
Indication: Alzheimer's Disease (AD)
Target: Unknown
Source Link: Link
Seelos Therapeutics, Inc.
Symbol: SEEL
Event Type: Trial Data - Top-Line Results
Event Phase: III
Drug: SLS-005
Disease Group: Neurology
Indication: Amyotrophic Lateral Sclerosis (ALS)
Target: TFEB Activator
Source Link: Link
Idorsia Pharmaceuticals Ltd
Symbol: IDIA
Event Type: Regulatory - Approval (U.S.)
Event Phase: Approved
Drug: TRYVIO
Disease Group: Cardiovascular
Indication: Hypertension (Systemic)
Target: Endothelin Receptor Type A (EDNRA), Endothelin Receptor Type B (EDNRB)
Source Link: Link
Cabaletta Bio, Inc.
Symbol: CABA
Event Type: Regulatory - Orphan Drug Designation (U.S.)
Event Phase: IND
Drug: CABA-201
Disease Group: Autoimmune/immunology
Indication: Systemic Sclerosis
Target: Autologous Chimeric Antigen Receptor T-cells (CAR-T), Cluster of Differentiation 19 (CD19)
Source Link: Link
Crinetics Pharmaceuticals, Inc.
Symbol: CRNX
Event Type: Trial Data - Top-Line Results
Event Phase: III
Drug: Paltusotine
Disease Group: Endocrine
Indication: Acromegaly
Target: Somatostatin Receptors
Source Link: Link
Bayer AG
Symbol: BAYN
Event Type: Trial Data - Top-Line Results
Event Phase: III
Drug: Elinzanetant
Disease Group: Endocrine
Indication: Menopause (including Hormone Replacement Therapy [HRT])
Target: Neurokinin Receptor
Source Link: Link
Merck & Co., Inc.
Symbol: MRK
Event Type: Trial Data - Top-Line Results
Event Phase: NDA/BLA
Drug: V116
Disease Group: Infectious Disease
Indication: Pneumococcal (Streptococcus pneumoniae) Vaccines (Antibacterial)
Target: Immune System, Streptococcus pneumoniae / Pneumococcus
Source Link: Link
Biogen, Inc.
Symbol: BIIB
Event Type: Trial Announcement - Trial Completed
Event Phase: Development Outside U.S.
Drug: BIIB110
Disease Group: Metabolic
Indication: Spinal Muscular Atrophy
Target: Activin A, Activin Receptor Type-2B (ACVR2B) (ACTRIIB)
Source Link: Link
Financing events
Acurx Pharmaceuticals (NAS: ACXP):
Description: Developing new antibiotics for infections caused by priority pathogens.
Deal Date: March 18, 2024.
Deal Type: Public Investment 2nd Offering.
Investors: Not specified.
Deal Size: Not specified.
Capstan Therapeutics:
Description: Developing RNA-based therapies for oncology and inflammatory diseases.
Deal Date: March 20, 2024.
Deal Type: Early Stage VC.
Investors: RA Capital Management, Novartis Venture Fund, Pfizer Ventures, and others.
Deal Size: $175 million.
Carlsmed:
Description: Developing personalized spine surgery platforms using machine learning.
Deal Date: March 18, 2024.
Deal Type: Later Stage VC.
Investors: US Venture Partners, B Capital Group, The Vertical Group, and others.
Deal Size: $52.5 million.
Celestial Therapeutics:
Description: Focused on dual-modal, broad-spectrum anti-infectives with anti-inflammatory activity.
Deal Date: June 22, 2023.
Deal Type: Seed Round.
Investors: StartX, Red Bear Angels, and others.
Deal Size: $1.23 million.
Chronos Therapeutics:
Description: Developing therapeutic drugs for nervous system disorders.
Deal Date: March 19, 2024.
Deal Type: Merger/Acquisition.
Acquirer: Evgen Pharma (LON: EVG).
Clasp Therapeutics:
Description: Developing cancer immunotherapies.
Deal Date: March 20, 2024.
Deal Type: Early Stage VC.
Investors: Catalio Capital Management, Third Rock Ventures, Novo Holdings, and others.
Deal Size: $150 million.
Cure51:
Description: Developing a clinical and molecular database for cancer drug discovery.
Deal Date: March 20, 2024.
Deal Type: Seed Round.
Investors: Sofinnova Partners, Hitachi Ventures, Kima Ventures, and others.
Deal Size: EUR 15 million.
Cybin (NEOE: CYBN):
Description: Developing psychedelic-based therapies for mental health issues.
Deal Date: March 19, 2024.
Deal Type: PIPE.
Investors: Sphera Healthcare, Deep Track Capital, RA Capital Management, and others.
Deal Size: $150 million.
Definitive Biotechnologies:
Description: Developing medical devices for point-of-care influenza diagnosis.
Deal Date: March 21, 2024.
Deal Type: Later Stage VC.
Investors: Undisclosed.
Deal Size: $900,000.
Delcath Systems (NAS: DCTH):
Description: Focused on the treatment of primary and metastatic liver cancers.
Deal Date: March 15, 2024.
Deal Type: PIPE.
Investors: Undisclosed.
Deal Size: $7 million.
Diatech Oncology:
Description: Developing molecular diagnostics for cancer treatment selection.
Deal Date: March 19, 2024.
Deal Type: Later Stage VC.
Investors: OrbiMed Advisors, Vivo Capital, Fidelity Management & Research Company, and others.
Deal Size: $45 million.
Eloxx Pharmaceuticals (NAS: ELOX):
Description: Developing therapies for genetic diseases caused by nonsense mutations.
Deal Date: March 15, 2024.
Deal Type: PIPE.
Investors: RTW Investments, Venrock Healthcare Capital Partners, Viking Global Investors, and others.
Deal Size: $50 million.
Ensysce Biosciences:
Description: Developing abuse-deterrent opioid analgesics.
Deal Date: March 21, 2024.
Deal Type: Later Stage VC.
Investors: Hermed Capital, Vivo Capital, Alexandria Venture Investments, and others.
Deal Size: $35 million.
Entasis Therapeutics (NAS: ETTX):
Description: Developing novel antibacterial therapies for multidrug-resistant Gram-negative infections.
Deal Date: March 19, 2024.
Deal Type: PIPE.
Investors: Redmile Group, Cormorant Asset Management, RTW Investments, and others.
Deal Size: $30 million.
Entrinsic Bio:
Description: Developing synthetic biology platforms for the production of cannabinoids.
Deal Date: March 20, 2024.
Deal Type: Early Stage VC.
Investors: Horizons Ventures, The Engine, Northpond Ventures, and others.
Deal Size: $20 million.
Ferrostat Biosciences:
Description: Developing small molecule therapeutics for cancer and neurodegenerative diseases.
Deal Date: March 20, 2024.
Deal Type: Early Stage VC.
Investors: Third Rock Ventures, Arch Venture Partners, F-Prime Capital, and others.
Deal Size: $50 million.
GenEdit:
Description: Developing gene editing technologies for the treatment of genetic diseases.
Deal Date: March 20, 2024.
Deal Type: Early Stage VC.
Investors: New Enterprise Associates, F-Prime Capital, Alexandria Venture Investments, and others.
Deal Size: $25 million.
GentiBio:
Description: Developing engineered regulatory T cells for autoimmune diseases.
Deal Date: March 19, 2024.
Deal Type: Series A.
Investors: RA Capital Management, Versant Ventures, Lightstone Ventures, and others.
Deal Size: $40 million.
Gilead Sciences (NAS: GILD):
Description: A biopharmaceutical company focused on infectious diseases and oncology.
Deal Date: March 20, 2024.
Deal Type: PIPE.
Investors: Undisclosed.
Deal Size: $150 million.
Gyroscope Therapeutics:
Description: Developing gene therapies for ocular diseases.
Deal Date: March 20, 2024.
Deal Type: Series D.
Investors: Forbion Capital Partners, Oxford Sciences Innovation, Syncona Investment Management, and others.
Deal Size: $200 million.
Reduction in force (RIF)
March 19 - CureVac: A company spokesperson confirmed that about 150 employees have agreed to a voluntary exit as the mRNA biotech cuts costs. The job losses stem from the company pivoting away from developing a COVID-19 vaccine. The news was first reported by German news outlet SWR.
Disease of the week
Myalgic Encephalomyelitis (ME), also known as Chronic Fatigue Syndrome (CFS), is a complex and debilitating chronic illness characterized by profound fatigue, post-exertional malaise, cognitive impairment, and a range of other symptoms. Here's a comprehensive overview:
Symptoms:
Fatigue: The hallmark symptom is extreme fatigue that doesn't improve with rest and may worsen with physical or mental exertion.
Post-Exertional Malaise (PEM): Physical or mental exertion can exacerbate symptoms, leading to a worsening of fatigue and other symptoms.
Cognitive Dysfunction: Often referred to as "brain fog," cognitive impairments include problems with memory, concentration, and word retrieval.
Sleep Disturbances: Despite fatigue, individuals may experience difficulties falling asleep or staying asleep, or they may wake up feeling unrefreshed.
Pain: Muscle pain, joint pain, headaches, and sore throat are common.
Orthostatic Intolerance: Symptoms worsen upon standing upright and may include dizziness, lightheadedness, or even fainting.
Autonomic Dysfunction: Dysregulation of the autonomic nervous system can lead to symptoms such as rapid heart rate, sweating abnormalities, and gastrointestinal issues.
Sensitivity to Stimuli: Some individuals may be hypersensitive to light, noise, smells, or certain foods.
Diagnosis:
Diagnosing ME/CFS can be challenging due to the absence of definitive diagnostic tests. It's typically diagnosed through:
Medical History: Physicians rely on the patient's medical history to identify symptoms and their duration.
Physical Examination: A physical exam helps rule out other potential causes of symptoms.
Diagnostic Criteria: The most commonly used criteria for diagnosing ME/CFS include the Fukuda criteria and the more recent International Consensus Criteria (ICC) and the Systemic Exertion Intolerance Disease (SEID) criteria.
Causes:
The exact cause of ME/CFS remains unknown, and it's likely that multiple factors contribute to its development. Potential contributors include:
Viral Infections: Some cases of ME/CFS begin following a viral infection, suggesting a possible trigger.
Immune Dysfunction: Abnormalities in the immune system have been observed in individuals with ME/CFS.
Central Nervous System Dysfunction: Dysregulation of the central nervous system may play a role in symptom development.
Genetic Predisposition: There may be a genetic component that increases susceptibility to developing ME/CFS.
Environmental Factors: Factors such as stress, toxins, or hormonal imbalances may contribute to the development of ME/CFS.
Treatment:
Currently, there is no cure for ME/CFS, and treatment primarily focuses on managing symptoms and improving quality of life. Treatment strategies may include:
Symptom Management: Medications may be prescribed to alleviate specific symptoms such as pain, sleep disturbances, or depression.
Lifestyle Modifications: Strategies such as pacing, prioritizing tasks, and balancing rest and activity can help manage symptoms.
Cognitive Behavioral Therapy (CBT): CBT can help individuals cope with the psychological impact of the illness and develop strategies for managing symptoms.
Graded Exercise Therapy (GET): This approach involves gradually increasing physical activity levels under the guidance of a healthcare professional.
Alternative Therapies: Some individuals find relief from symptoms through complementary and alternative therapies such as acupuncture, massage, or dietary changes.
Research and Controversies:
ME/CFS remains an area of active research, but controversies persist regarding its etiology, diagnosis, and treatment. Some of the key areas of research include:
Biomarkers: Identifying biological markers that can aid in diagnosis and monitoring disease progression.
Immunological Research: Understanding the role of the immune system in ME/CFS and identifying potential targets for treatment.
Neurological Dysfunction: Investigating abnormalities in the central nervous system and their contribution to symptom development.
Treatment Trials: Conducting clinical trials to evaluate the efficacy of different treatment approaches, including medications, therapies, and lifestyle interventions.
What I’ve read this week
*Click on the pic to read*