Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
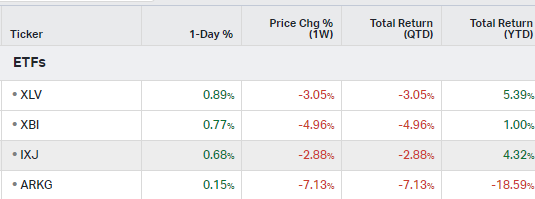
How the market performed this week
ETFs
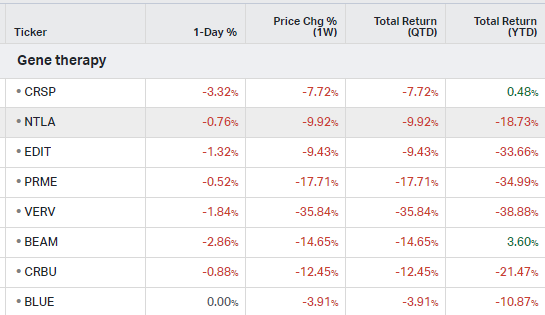
Gene Therapy
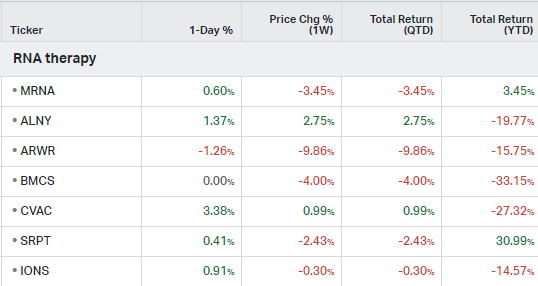
RNA Therapy
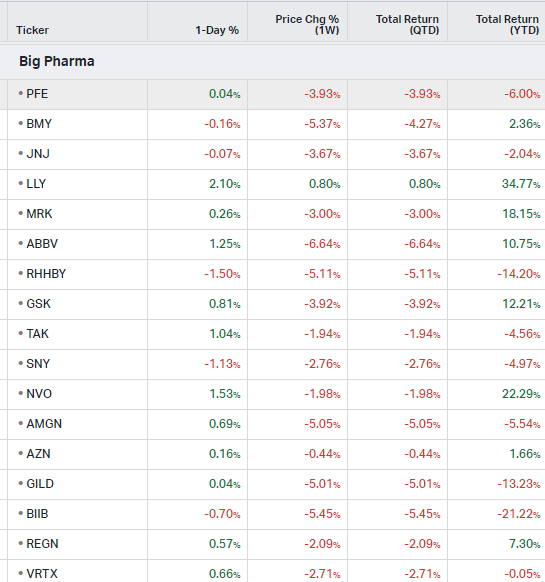
Big Pharma
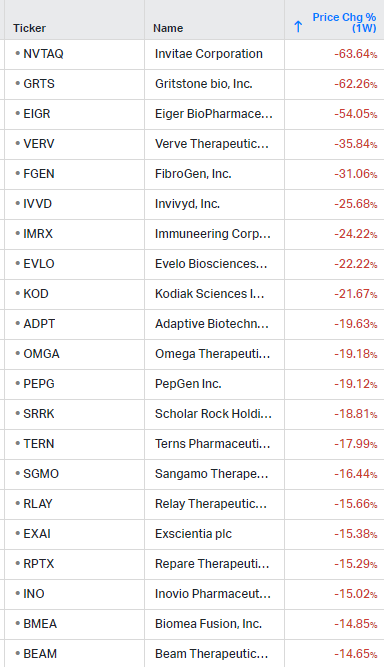
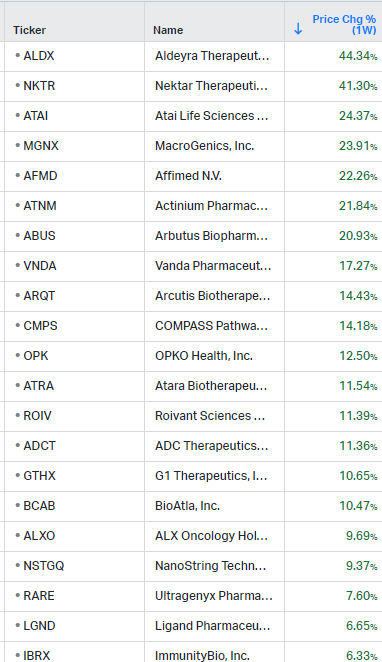
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
XOMA Corporation (XOMA)
Event Phase: I
Drug: KIN-3248
Disease Group: Oncology
Indication: Solid Tumors, Biliary Tract Cancer
Target: Fibroblast Growth Factor Receptor (FGFR)
LOA: 5%
Source Link: GlobeNewswire
Event Phase: IND
Drug: KIN-7136
Disease Group: Oncology
Indication: Solid Tumors
Target: Mitogen-activated ERK kinase (MEK, MAPKK, MAP2K)
Source Link: GlobeNewswire
Preclinical
Drug: KIN-8741
Disease Group: Oncology
Indication: Solid Tumors
Target: Hepatocyte growth factor receptor (c-Met, HGFR)
Source Link: GlobeNewswire
Preclinical
Drug: KIN004
Disease Group: Oncology
Indication: Solid Tumors
Target: Cyclin Dependent Kinase 12 (CDK-12)
Source Link: GlobeNewswire
Kintara Therapeutics (KTRA)
Preclinical
Drug: VAL-083
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: DNA Damage Repair, DNA synthesis, Protein synthesis, RNA synthesis
Source Link: Accesswire
Development Outside U.S.
Drug: VAL-083
Disease Group: Oncology
Indication: Chronic Myelogenous Leukemia (CML)
Target: DNA Damage Repair, DNA synthesis, Protein synthesis, RNA synthesis
Source Link: Accesswire
III
Drug: VAL-083
Disease Group: Oncology
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Target: DNA Damage Repair, DNA synthesis, Protein synthesis, RNA synthesis
LOA: 44%
Source Link: Accesswire
III
Drug: REM-001
Disease Group: Oncology
Indication: Breast Cancer
Target: Reactive Oxygen Species/Free Radicals
LOA: 44%
Source Link: Accesswire
II
Drug: REM-001
Disease Group: Oncology
Indication: Skin Cancer - Basal Cell Carcinoma Nevus Syndrome (BCCNS) / Gorlin Syndrome
Target: Reactive Oxygen Species/Free Radicals
LOA: 11%
Source Link: Accesswire
IND
Drug: VAL-083
Disease Group: Oncology
Indication: Ovarian Cancer
Target: DNA Damage Repair, DNA synthesis, Protein synthesis, RNA synthesis
Source Link: Accesswire
CytomX Therapeutics, Inc. (CTMX)
Preclinical
Drug: Probody T-Cell Therapy (CytomX)
Disease Group: Oncology
Indication: Cancer
Target: Cluster of Differentiation 3 (CD3), Immune System
Source Link: GlobeNewswire
Ipsen SA (IPSEY)
Preclinical
Drug: STRO-003
Disease Group: Oncology
Indication: Solid Tumors
Target: ROR-1/NTRKR1
Source Link: GlobeNewswire
Appili Therapeutics Inc. (APLI)
Approved
Drug: LIKMEZ
Disease Group: Infectious Disease
Indication: Clostridium difficile-Associated Diarrhea/Infection (CDAD/CDI)
Target: Microbial DNA, Reactive Oxygen Species/Free Radicals
LOA: 100%
Source Link: BusinessWire
III
Drug: ATI-1801
Disease Group: Infectious Disease
Indication: Leishmaniasis
Target: Unknown
LOA: 59%
Source Link: BusinessWire
Preclinical
Drug: ATI-1701
Disease Group: Infectious Disease
Indication: Antibacterial - General
Target: Unknown
Source Link: BusinessWire
Dr. Reddy's Laboratories Ltd. (RDY)
III
Drug: Favipiravir
Disease Group: Infectious Disease
Indication: Influenza (excluding vaccines)
Target: RNA polymerase, SARS-CoV-2
LOA: 59%
Source Link: BusinessWire
II
Drug: Favipiravir
Disease Group: Infectious Disease
Indication: COVID-19 Prevention
Target: RNA polymerase, SARS-CoV-2
LOA: 23%
Source Link: BusinessWire
Development Outside U.S.
Drug: Favipiravir
Disease Group: Infectious Disease
Indication: Antiviral - Other Treatments
Target: RNA polymerase, SARS-CoV-2
Source Link: BusinessWire
AbbVie Inc. (ABBV)
Approved
Drug: Enablex
Disease Group: Urology
Indication: Overactive Bladder (OAB)
Target: Muscarinic acetylcholine receptor
LOA: 100%
Source Link: PR Newswire
ANI Pharmaceuticals, Inc. (ANIP)
Approved
Drug: Vancocin
Disease Group: Infectious Disease
Indication: Clostridium difficile-Associated Diarrhea/Infection (CDAD/CDI)
Target: Cell wall synthesis
LOA: 100%
Source Link: PR Newswire
Amgen, Inc. (AMGN)
Approved
Drug: Vimovo
Disease Group: Neurology
Indication: Arthritis Pain
Target: Cyclooxygenases (COX-1, COX-2, and COX-3), Proton pump
LOA: 100%
Source Link: PR Newswire
Approved
Drug: Pennsaid 2%
Disease Group: Neurology
Indication: Osteoarthritis Pain
Target: Cyclooxygenases (COX-1, COX-2, and COX-3)
LOA: 100%
Source Link: PR Newswire
Novartis AG (NVS)
Approved
Drug: Fiorinal with Codeine
Disease Group: Neurology
Indication: Tension Headache
Target: Adenosine Receptors, Cyclooxygenases (COX-1, COX-2, and COX-3), GABA-A Receptor
LOA: 100%
Source Link: PR Newswire
Approved
Drug: Visken
Disease Group: Cardiovascular
Indication: Hypertension (Systemic), Angina
Target: Beta Adrenergic Receptors
LOA: 100%
Source Link: PR Newswire
Mayne Pharma Group Limited (MYX:AU)
Approved
Drug: Estelle
Disease Group: Endocrine
Indication: Contraception
Target: Estrogen Receptor Alpha (ER1 or ER alpha), Estrogen Receptor Beta (ER2 or ER beta)
LOA: 100%
Source Link: PR Newswire
Development Outside U.S.
Drug: Estelle
Disease Group: Obstetrics/Gynecology
Indication: Dysmenorrhea
Target: Estrogen Receptor Alpha (ER1 or ER alpha), Estrogen Receptor Beta (ER2 or ER beta)
Source Link: PR Newswire
Relief Therapeutics Holding AG (RLF)
Approved
Drug: Cambia
Disease Group: Neurology
Indication: Migraine and Other Headaches
Target: Cyclooxygenases (COX-1, COX-2, and COX-3)
LOA: 100%
Source Link: PR Newswire
RVL Pharmaceuticals plc (RVLP)
Approved
Drug: ConZip
Disease Group: Neurology
Indication: Chronic Pain
Target: Opioid receptors
LOA: 100%
Source Link: PR Newswire
Sol-Gel Technologies, Ltd. (SLGL)
Approved
Drug: Epsolay
Disease Group: Dermatology
Indication: Rosacea
Target: Bacteria-miscellaneous, Retinoic acid receptor (RARs)
LOA: 100%
Source Link: PR Newswire
Approved
Drug: Twyneo
Disease Group: Dermatology
Indication: Acne
Target: Retinoic acid receptor (RARs)
LOA: 100%
Source Link: PR Newswire
Hikma Pharmaceuticals plc (HIK)
Development Outside U.S.
Drug: Blexten
Disease Group: Allergy
Indication: Allergic Rhinitis, Urticaria, Pruritus, Atopic Dermatitis (Eczema)
Target: Histamine H1 Receptor (HRH1)
Source Link: PR Newswire
Mithra Pharmaceuticals SA (MITRA)
III
Drug: Donesta
Disease Group: Endocrine
Indication: Menopause (including Hormone Replacement Therapy [HRT])
Target: Estrogen Receptor Alpha (ER1 or ER alpha), Estrogen Receptor Beta (ER2 or ER beta)
LOA: 63%
Source Link: PR Newswire
Preclinical
Drug: Donesta
Disease Group: Dermatology, Neurology
Indication: Wound Healing, Neurology - Other
Target: Estrogen Receptor Alpha (ER1 or ER alpha), Estrogen Receptor Beta (ER2 or ER beta)
Source Link: PR Newswire
BioInvent International AB (BINV)
II
Drug: BI-1910
Disease Group: Oncology
Indication: Solid Tumors
Target: Immune System, TNFR (TNF Receptor)/CD120
LOA: 11%
Source Link: Accesswire
Traws Pharma, Inc. (TRAW)
II
Drug: Estybon (Oral)
Disease Group: Oncology
Indication: Head and Neck Cancer, Melanoma, Non-Small Cell Lung Cancer (NSCLC)
Target: PI3K/AKT pathway, Polo-like kinase 1 (Plk1)
LOA: 11%
Source Link: GlobeNewswire
II
Drug: Estybon (Intravenous)
Disease Group: Oncology
Indication: Head and Neck Cancer
Target: PI3K/AKT pathway, Polo-like kinase 1 (Plk1)
LOA: 11%
Source Link: GlobeNewswire
IND
Drug: Narazaciclib
Disease Group: Oncology
Indication: Mantle Cell Lymphoma - NHL
Target: AMP-activated protein kinase (AMPK), Cyclin Dependent Kinase 4 (CDK-4), Cyclin Dependent Kinase 6 (CDK-6)
Source Link: GlobeNewswire
I
Drug: Narazaciclib
Disease Group: Oncology
Indication: Solid Tumors
Target: AMP-activated protein kinase (AMPK), Cyclin Dependent Kinase 4 (CDK-4), Cyclin Dependent Kinase 6 (CDK-6)
LOA: 5%
Source Link: GlobeNewswire
II
Drug: Narazaciclib
Disease Group: Oncology
Indication: Uterine (Endometrial) Cancer
Target: AMP-activated protein kinase (AMPK), Cyclin Dependent Kinase 4 (CDK-4), Cyclin Dependent Kinase 6 (CDK-6)
LOA: 11%
Source Link: GlobeNewswire
I
Drug: TRX-01
Disease Group: Infectious Disease
Indication: COVID-19 Treatment
Target: SARS-CoV-2
LOA: 13%
Source Link: GlobeNewswire
I
Drug: TRX100
Disease Group: Infectious Disease
Indication: Influenza (excluding vaccines)
Target: Influenza Virus
LOA: 13%
Source Link: GlobeNewswire
Incyte Corporation (INCY)
II
Drug: INCB54707
Disease Group: Respiratory, Allergy
Indication: Asthma, Pruritus, Urticaria
Target: JAK/STAT
LOA: 18%
Source Link: BusinessWire
III
Drug: INCB54707
Disease Group: Dermatology
Indication: Hidradenitis Suppurativa, Vitiligo
Target: JAK/STAT
LOA: 69% - 70%
Source Link: BusinessWire
Clinical trials (LOA=likelihood of approval)
Biora Therapeutics, Inc. (BIOR)
Trial Name: Phase I - FIH (SAD/MAD)
Drug: BT-600
Disease Group: Autoimmune/immunology
Indication: Ulcerative Colitis (UC)
Lead Indication: Y
Target: JAK/STAT
LOA: 11%
Source Link: Biomedtracker
Candel Therapeutics, Inc. (CADL)
Trial Name: Phase I/II - PaTK02
Drug: Aglatimagene Besadenovec
Disease Group: Oncology
Indication: Pancreatic Cancer
Lead Indication: N
Target: Thymidine Kinase, Tumor Cells
LOA: 11%
Source Link: GlobeNewswire
LENZ Therapeutics (LENZ)
Trial Name: Phase III - CLARITY 1, Phase III - CLARITY 2 w/LNZ101, Phase III - CLARITY 3
Drug: LNZ-100
Disease Group: Ophthalmology
Indication: Presbyopia
Lead Indication: Y
Target: Muscarinic acetylcholine receptor
LOA: 64%
Source Link: BusinessWire
Basilea Pharmaceutica Ltd. (BSLN)
Drug: Zeftera
Disease Group: Infectious Disease
Indication: Septicemia or Bacteremia (Antibacterial, including Endocarditis)
Lead Indication: N
Target: Penicillin Binding Proteins (PBP)
LOA: 100%
Source Link: GlobeNewswire
Travere Therapeutics, Inc. (TVTX)
Trial Name: Phase III - PROTECT Open-Label Extension (OLE)
Drug: Filspari
Disease Group: Renal
Indication: Immunoglobulin A (IgA) Nephropathy (Berger's Disease)
Lead Indication: Y
Target: Angiotensin II Receptor Type 1 (AT1), Endothelin Receptor Type A (EDNRA)
LOA: 100%
Source Link: Kireports
MacroGenics, Inc. (MGNX)
Trial Name: Phase II - TAMARACK
Drug: MGC018
Disease Group: Oncology
Indication: Prostate Cancer
Lead Indication: N
Target: B7-H3
LOA: 11%
Source Link: GlobeNewswire
VBI Vaccines Inc. (VBIV)
Trial Name: Phase I/IIa/b - Dose Escalation
Drug: VBI-1901
Disease Group: Oncology
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Lead Indication: Y
Target: Human Cytomegalovirus (HCMV), Immune System
LOA: 11%
Source Link: BusinessWire
Moderna, Inc. (MRNA)
Trial Name: Phase I/II - Paramount (Pediatric)
Drug: mRNA-3927
Disease Group: Metabolic
Indication: Mitochondrial Respiratory-Chain Diseases
Lead Indication: Y
Target: Propionyl-CoA carboxylase
LOA: 25%
Source Link: AccessWire
Zentalis Pharmaceuticals (ZNTL)
Trial Name: Preclinical Studies
Drug: ZN-c3
Disease Group: Oncology
Indication: Solid Tumors
Lead Indication: N
Target: Wee1 Inhibitor
LOA: 11%
Source Link: GlobeNewswire
Kiniksa Pharmaceuticals Corporation (KNSA)
Trial Name: Phase II - Proof-of-Concept
Drug: Abiprubart
Disease Group: Autoimmune/immunology
Indication: Rheumatoid Arthritis (RA)
Lead Indication: Y
Target: Cluster of Differentiation 40 (CD40)
LOA: 19%
Source Link: GlobeNewswire
Traws Pharma, Inc. (TRAW)
Trial Name: Phase I - PK/PD Study (Healthy Volunteers)
Drug: TRX100
Disease Group: Infectious Disease
Indication: Influenza (excluding vaccines)
Lead Indication: Y
Target: Influenza Virus
LOA: 13%
Source Link: GlobeNewswire
FibroGen, Inc. (FGEN)
Trial Name: Phase I - 001 (mCRPC)
Drug: FG-3246
Disease Group: Oncology
Indication: Prostate Cancer
Lead Indication: Y
Target: Cluster of Differentiation 46 (CD46)
LOA: 11%
Source Link: GlobeNewswire
Vanda Pharmaceuticals, Inc. (VNDA)
Drug: Fanapt
Disease Group: Psychiatry
Indication: Bipolar Disorder
Lead Indication: N
Target: Alpha 1 Adrenergic Receptor , Dopamine 2 (D2) Receptor, Dopamine 3 (D3) Receptor, Dopamine 4 (D4) Receptor, Serotonin 5-HT2A receptor, Serotonin 5-HT2B receptor, Serotonin 5-HT6 receptor
LOA: 100%
Source Link: PR Newswire
Cantargia AB (CANTA)
Trial Name: Preclinical Studies
Drug: CAN10
Disease Group: Cardiovascular
Indication: Atherosclerosis
Lead Indication: N
Target: IL-1 (Interleukin-1), IL-33 (Interleukin-33)IL-33 Receptor, IL-36/IL-36R (Interleukin-36/receptor)
Source Link: AccessWire
Kiromic BioPharma, Inc. (KRBP)
Trial Name: Phase I - First-in-Human
Drug: Deltacel
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: N
Target: Unknown
LOA: 5%
Source Link: Kiromic
Evaxion Biotech A/S (EVAX)
Trial Name: Preclinical Studies
Drug: EVX-B1
Disease Group: Infectious Disease
Indication: Staphylococcal Vaccines and Other Staphylococcus-Specific Agents (Antibacterial)
Lead Indication: N
Target: Staphylococcus
Source Link: GlobeNewswire
Mira Pharmaceuticals, Inc. (MIRA)
Trial Name: Preclinical Studies
Drug: Ketamir-2
Disease Group: Psychiatry
Indication: Major Depressive Disorder (MDD)
Lead Indication: N
Target: NMDA Glutamate Receptor
Source Link: PR Newswire
Mira Pharmaceuticals, Inc. (MIRA)
Trial Name: Preclinical Studies
Drug: Ketamir-2
Disease Group: Psychiatry
Indication: Post-Traumatic Stress Disorder (PTSD)
Lead Indication: N
Target: NMDA Glutamate Receptor
Source Link: PR Newswire
Equillium, Inc. (EQ)
Trial Name: Phase Ib - EQUALISE
Drug: EQ001
Disease Group: Autoimmune/immunology
Indication: Lupus Nephritis
Lead Indication: N
Target: Cluster of Differentiation 6 (CD6)
LOA: 11%
Source Link: BusinessWire
Gritstone bio, Inc. (GRTS)
Trial Name: Phase II/III - GRANITE-CRC-1L
Drug: GRANITE
Disease Group: Oncology
Indication: Colorectal Cancer (CRC)
Lead Indication: Y
Target: Immune System, Tumor Cells
LOA: 49%
Source Link: GlobeNewswire
Actinium Pharmaceuticals, Inc. (ATNM) (continued)
Lead Indication: Y
Target: Cluster of Differentiation 45 (CD45)/Lymphocyte Common Antigen (LCA)
LOA: 49%
Source Link: PR Newswire
Biomea Fusion, Inc. (BMEA)
Trial Name: Phase II - COVALENT-112 (Canada & US)
Drug: BMF-219
Disease Group: Endocrine
Indication: Diabetes Mellitus, Type I
Lead Indication: N
Target: Menin
LOA: 15%
Source Link: GlobeNewswire
Novavax, Inc. (NVAX)
Trial Name: Phase II/III - Vaccine Booster Dose
Drug: Nuvaxovid
Disease Group: Infectious Disease
Indication: COVID-19 Prevention
Lead Indication: Y
Target: Immune System, Influenza Virus, SARS-CoV-2
LOA: 99%
Source Link: PR Newswire
Disc Medicine, Inc. (IRON)
Trial Name: Phase II - AURORA
Drug: Bitopertin
Disease Group: Metabolic
Indication: Porphyria
Lead Indication: N
Target: Glycine Neurotransmitter Transporter (GlyT-1)
LOA: 28%
Source Link: GlobeNewswire
AstraZeneca PLC (AZN)
Drug: Danicopan
Disease Group: Autoimmune/immunology
Indication: Paroxysmal Nocturnal Hemoglobinuria (PNH)
Lead Indication: N
Target: Factor D (alternate complement pathway)
LOA: 100%
Source Link: BusinessWire
Surrozen Inc. (SRZN)
Trial Name: Phase Ia - SAD/MAD Study (Australia)
Drug: SZN-043
Disease Group: Endocrine
Indication: Alcoholic Liver Disease / Alcoholic Hepatitis
Lead Indication: Y
Target: Asialoglycoprotein receptor (ASGR), WNT Signaling Pathway
Source Link: GlobeNewswire
Financing events
Reduction in force (RIF)
April 4 - Amylyx Pharmaceuticals: After Relyvrio failed a confirmatory trial, Amylyx has pulled the ALS therapy off the market and is laying off about 70% of staff. As of the end of 2023, Amylyx had 384 full-time employees, according to an annual securities filing. The cuts will leave the company with about 100 remaining workers. Story
April 1 - Carisma Therapeutics: The immunotherapy-focused biotech plans to let go of 37% of staffers and drop one of two clinical assets in an effort to stretch its cash into the third quarter of 2025. The layoffs follow a reverse merger with Sesen Bio in March 2023, a move that increased Carisma's personnel costs. Story
Disease of the week
Erdheim-Chester Disease (ECD) is an extremely rare form of non-Langerhans cell histiocytosis, a group of disorders characterized by the excessive production and accumulation of histiocytes, a type of white blood cell, in various tissues and organs of the body. ECD specifically involves the infiltration of histiocytes into multiple organs, leading to the formation of tumors or lesions.
Here are some key points about Erdheim-Chester Disease:
Epidemiology: ECD is an exceedingly rare disease, with only a few hundred cases reported worldwide. It typically affects adults between the ages of 40 and 70, although cases have been reported in individuals of all ages, including children.
Clinical Presentation: The clinical presentation of ECD can vary widely depending on the organs involved. Common symptoms may include bone pain, especially in the long bones of the legs, as well as fatigue, weight loss, fever, and neurological deficits if the central nervous system is affected. Other organs commonly affected include the kidneys, heart, lungs, skin, and retroperitoneum (the area behind the abdominal cavity).
Pathophysiology: The exact cause of ECD is unknown. However, it is believed to be related to the abnormal proliferation and activation of histiocytes, leading to the formation of granulomatous lesions in various tissues. Recent research suggests that somatic mutations in genes such as BRAF, MAP2K1, and PIK3CA may play a role in the pathogenesis of ECD.
Diagnosis: Diagnosis of ECD can be challenging due to its rarity and nonspecific symptoms. It often involves a combination of clinical evaluation, imaging studies (such as X-rays, CT scans, and MRI), and histopathological examination of biopsy samples taken from affected tissues.
Treatment: There is no standard treatment protocol for ECD due to its rarity and variability in clinical presentation. Treatment options may include systemic corticosteroids, chemotherapy agents (such as interferon-alpha or cladribine), targeted therapies (such as BRAF inhibitors for patients with BRAF mutations), and immunomodulatory agents. In some cases, surgical intervention may be necessary to address complications such as bone fractures or obstructive lesions.
Prognosis: The prognosis of ECD varies depending on the extent and severity of organ involvement. Some patients may have a relatively indolent course with stable disease over many years, while others may experience rapid progression and significant morbidity or mortality. Close monitoring by a multidisciplinary team of healthcare providers is essential for managing the disease and its complications.
What I’ve read this week
*Click on the pic to read*