Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
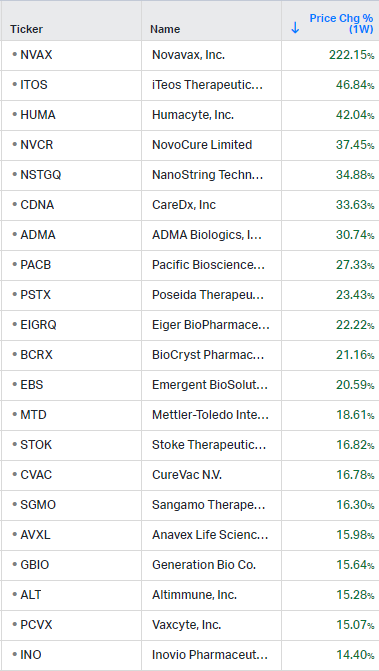
How the market performed this week
ETFs
Gene Therapy
RNA Therapy
Big Pharma
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Astellas Pharma, Inc.
Symbol: 4503:JP
Event Phase: Approved
Drug: Cresemba
Indication: Aspergillosis
Target: Cytochrome p450
LOA: 100%
Source Link: GlobeNewswire
Sol-Gel Technologies, Ltd.
Symbol: SLGL
Event Phase: Approved
Drug: Twyneo
Indication: Acne
Target: Retinoic acid receptor (RARs)
LOA: 100%
Source Link: GlobeNewswire
Anika Therapeutics, Inc.
Symbol: ANIK
Event Phase: III
Drug: Cingal
Indication: Osteoarthritis Pain
Target: Glucocorticoid Receptor (GR)
LOA: 52%
Source Link: Newswire
Erasca, Inc.
Symbol: ERAS
Event Phase: Preclinical
Drug: ERAS-0015
Indication: Solid Tumors
Target: Ras
LOA: N/A
Source Link: GlobeNewswire
NeuroSense Therapeutics Ltd.
Symbol: NRSN
Event Phase: I
Drug: PrimeC
Indication: Amyotrophic Lateral Sclerosis (ALS)
Target: Cyclooxygenase 2 (COX2) / Prostaglandin-Endoperoxide Synthase 2 (PTGS2), Topoisomerase II (DNA gyrase) and IV
LOA: 6%
Source Link: PRNewswire
AC Immune SA
Symbol: ACIU
Event Phase: II
Drug: ACI-24
Indication: Alzheimer's Disease (AD)
Target: Amyloid Beta/Amyloid Plaques, Immune System
LOA: 13%
Source Link: BusinessWire
AC Immune SA
Symbol: ACIU
Event Phase: I
Drug: ACI-24
Indication: Down Syndrome
Target: Amyloid Beta/Amyloid Plaques, Immune System
LOA: 6%
Source Link: BusinessWire
Fulcrum Therapeutics Inc.
Symbol: FULC
Event Phase: III
Drug: Losmapimod
Indication: Facioscapulohumeral Muscular Dystrophy (FSHD)
Target: p38 MAP kinase (MAPK)
LOA: 55%
Source Link: GlobeNewswire
Clinical trials (LOA=likelihood of approval)
Roche Holding AG
Symbol: RHHBY
Event Type: Trial Data - Updated Results
Event Phase: II
Drug: CT-388
Indication: Obesity
Target: GIP Receptor (GIPR)/Glucose-Dependent Insulinotropic Polypeptide Receptor, GLP-1 Receptor
LOA: 25%
Source Link: Business Wire
Roche Holding AG
Symbol: RHHBY
Event Type: Trial Data - Updated Results
Event Phase: II
Drug: CT-388
Indication: Diabetes Mellitus, Type II
Target: GIP Receptor (GIPR)/Glucose-Dependent Insulinotropic Polypeptide Receptor, GLP-1 Receptor
LOA: 15%
Source Link: Business Wire
AstraZeneca PLC
Symbol: AZN
Event Type: Trial Data - Updated Results
Event Phase: III
Drug: Sipavibart
Indication: COVID-19 Prevention
Target: SARS-CoV-2
LOA: 59%
Source Link: AstraZeneca
Arvinas, Inc.
Symbol: ARVN
Event Type: Trial Data - Updated Results
Event Phase: III
Drug: Vepdegestrant
Indication: HR+/HER2- Breast Cancer
Target: Estrogen Receptor Alpha (ER1 or ER alpha), Estrogen Receptor Beta (ER2 or ER beta)
LOA: 49%
Source Link: GlobeNewswire
Biogen, Inc.
Symbol: BIIB
Event Type: Trial Data - Suspension
Event Phase: Suspended
Drug: BIIB105
Indication: Amyotrophic Lateral Sclerosis (ALS)
Target: Ataxin-2
LOA:
Source Link: GlobeNewswire
Ionis Pharmaceuticals, Inc.
Symbol: IONS
Event Type: Trial Data - Updated Results
Event Phase: II
Drug: ION-581
Indication: Angelman Syndrome
Target: E3 ubiquitin ligase
LOA: 12%
Source Link: PR Newswire
Eli Lilly and Company
Symbol: LLY
Event Type: Trial Data - Top-Line Results
Event Phase: III
Drug: Efsitora alfa
Indication: Diabetes Mellitus, Type II
Target: Insulin Receptor
LOA: 58%
Source Link: PR Newswire
Avenue Therapeutics Inc.
Symbol: ATXI
Event Type: Trial Announcement - Trial Completed
Event Phase: II
Drug: AJ201
Indication: Spinal Muscular Atrophy
Target: NRF2
LOA: 25%
Source Link: GlobeNewswire
Amgen, Inc.
Symbol: AMGN
Event Type: Regulatory - Accelerated/Conditional Approval (U.S.)
Event Phase: Approved
Drug: Imdelltra
Indication: Small Cell Lung Cancer (SCLC)
Target: Cluster of Differentiation 3 (CD3), Delta-like 3 (DLL3)
LOA: 100%
Source Link: PR Newswire
Bristol Myers Squibb Company
Symbol: BMY
Event Type: Trial Data - Updated Results
Event Phase: Approved
Drug: Sotyktu
Indication: Psoriasis
Target: JAK/STAT , Tyrosine kinase 2 (TYK2)
LOA: 100%
Source Link: Business Wire
Nuvalent, Inc.
Symbol: NUVL
Event Type: Regulatory - Breakthrough Therapy Designation (U.S.)
Event Phase: II
Drug: NVL-655
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Anaplastic lymphoma kinase (ALK)
LOA: 11%
Source Link: PR Newswire
Bayer AG
Symbol: BAYN
Event Type: Trial Data - Updated Results
Event Phase: III
Drug: Elinzanetant
Indication: Menopause (including Hormone Replacement Therapy [HRT])
Target: Neurokinin Receptor
LOA: 63%
Source Link: Business Wire
Olema Pharmaceuticals, Inc.
Symbol: OLMA
Event Type: Trial Data - Updated Results
Event Phase: III
Drug: Palazestrant
Indication: HR+/HER2- Breast Cancer
Target: Estrogen Receptor Alpha (ER1 or ER alpha), Selective Estrogen Receptor Degrader (SERD)
LOA: 46%
Source Link: GlobeNewswire
Travere Therapeutics, Inc.
Symbol: TVTX
Event Type: Trial Data - Updated Results
Event Phase: Approved
Drug: Filspari
Indication: Immunoglobulin A (IgA) Nephropathy (Berger's Disease)
Target: Angiotensin II Receptor Type 1 (AT1), Endothelin Receptor Type A (EDNRA)
LOA: 100%
Source Link: PR Newswire
Kymera Therapeutics, Inc. (KYMR)
Trial Data: Updated Results
Phase: I
Drug: KT-333
Indication: Solid Tumors
Target: STAT3 Transcription Factor
Response Rate: 5%
BioInvent International AB (BINV)
Trial Data: Updated Results
Phase: II
Drug: BI-1206
Indication: Non-Hodgkin's Lymphoma (NHL)
Target: Fc receptors, Immune System
Response Rate: 11%
Actinium Pharmaceuticals, Inc. (ATNM)
Trial Data: Updated Results
Phase: III
Drug: Iomab-B
Indication: Acute Myelogenous Leukemia (AML)
Target: Cluster of Differentiation 45 (CD45)/Lymphocyte Common Antigen (LCA)
Response Rate: 49%
NanoViricides, Inc. (NNVC)
Trial Data: Preclinical Results
Phase: Preclinical
Drug: NV-CoV-2
Indication: Antiviral - Other Treatments
Target: SARS-CoV-2
Pliant Therapeutics, Inc. (PLRX)
Trial Data: Top-Line Results
Phase: II
Drug: Bexotegrast
Indication: Idiopathic Pulmonary Fibrosis (IPF)
Target: Integrin Alpha-V Family, Integrin beta-1/CD29
Response Rate: 13%
Unicycive Therapeutics, Inc. (UNCY)
Trial Data: Updated Results
Phase: II
Drug: Renazorb
Indication: Hyperphosphatemia
Target: Phosphate
Response Rate: 15%
Novo Nordisk A/S (NVO)
Trial Data: Top-Line Results
Phase: III
Drug: Mim8
Indication: Hemophilia A
Target: Coagulation Factor IX, Coagulation Factor X
Response Rate: 78%
Merus N.V. (MRUS)
Regulatory: Breakthrough Therapy Designation (U.S.)
Phase: II
Drug: Petosemtamab
Indication: Head and Neck Cancer
Target: EGFR (Epidermal Growth Factor Receptor), LGR5 (Leucine-rich repeat-containing G-protein coupled receptor 5)
Response Rate: 11%
Valneva SE (VLA)
Trial Data: Updated Results
Phase: Approved
Drug: Ixchiq
Indication: Chikungunya Virus
Target: Immune System
Response Rate: 100%
Financing events
AATec Medical
Description: Developer of a multi-product platform for treating inflammatory diseases and viral infections.
Verticals: HealthTech, Life Sciences
Deal Date: 14-May-2024
Deal Type: Early Stage VC
Deal Synopsis: Raising EUR 15 million of Series A venture funding.
Deal Size: EUR 16.07 million
Ajax Therapeutics
Description: Developer of novel therapies targeting cytokine signaling pathways for hematologic malignancies.
Verticals: Life Sciences, Oncology
Deal Date: 13-May-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $95 million of Series C venture funding led by Goldman Sachs Asset Management.
Investors: Eli Lilly, Goldman Sachs, RA Capital Management, Schrodinger
Deal Size: $95 million
Allogene Therapeutics (NAS: ALLO)
Description: Clinical-stage biotech specializing in immuno-oncology and allogeneic T-cell products.
Verticals: Life Sciences, Oncology
Deal Date: 14-May-2024
Deal Type: Public Investment 2nd Offering
Deal Synopsis: Raised $110 million in a public offering on Nasdaq.
Deal Size: $110 million
ArgusEye
Description: Developer of sensor technology for real-time detection of biological systems and processes.
Verticals: Life Sciences
Deal Date: 13-May-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised EUR 2.8 million led by Eir Ventures and Voima Ventures.
Investors: Eir Ventures, Voima Ventures
Deal Size: EUR 3 million
BriaCell Therapeutics (TSE: BCT)
Description: Immuno-oncology-focused biotech company developing treatments for cancer.
Verticals: Life Sciences, Manufacturing, Oncology
Deal Date: 15-May-2024
Deal Type: PIPE
Deal Synopsis: In talks to receive CAD 5 million for a private placement.
Deal Size: CAD 3.65 million
Brixton Biosciences
Description: Developer of therapies for chronic and acute pain.
Verticals: Life Sciences
Deal Date: 14-May-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $33 million of Series B venture funding led by Schooner Capital.
Investors: Catalyst Health Ventures, Excelestar Ventures, PV Capital Management, Schooner Capital, Sparta Group, SV Health Investors
Deal Size: $33 million
Cellbox Labs
Description: Developer of miniature organ replicas for pharmaceutical preclinical tests.
Verticals: Life Sciences, Oncology
Deal Date: 13-May-2024
Deal Type: Seed Round
Deal Synopsis: Raised EUR 935,000 led by Buildit.
Investors: ASP Asset Management, Buildit, LatBan
Deal Size: EUR 1 million
Elegen
Description: Developer of microfluidics technology for synthetic biology.
Verticals: Life Sciences
Deal Date: 14-May-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $35 million through Series B, B-1, and B-2 funding led by Triatomic Capital.
Investors: 8VC, ACVC Partners, Agilent Technologies, AME Cloud Ventures, Andreessen Horowitz, Digitalis Ventures, GSK, John Ballantyne, KdT Ventures, Triatomic Capital
Deal Size: $35 million
Entactbio
Description: Biotechnology research company enhancing beneficial proteins.
Verticals: Life Sciences
Deal Date: 15-May-2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $40.32 million of Series A venture funding.
Deal Size: $40.32 million
Reduction in force (RIF)
May 16 - Akari Therapeutics: The biotech is implementing a 67% workforce reduction in efforts to reduce operating costs. Part of the restructuring plan includes eliminating certain undisclosed senior management positions. Release
May 15 - BioMarin: After sharing plans to slash several pipeline programs, BioMarin has confirmed that 170 employees are also leaving as part of the R&D restructure. The job cuts represent around 5% of the company’s workforce, a BioMarin spokesperson told Fierce Biotech via email. The layoffs are slated to be complete by the end of July. Story
May 15 - Tenaya Therapeutics: Heart disease-focused Tenaya is implementing “cost-containment measures” designed to fund clinical readouts for two lead gene therapies. As a result, the biotech is cutting down its workforce by 22%, with the changes due to be completed in the coming weeks. Story
May 15 - Bolt Biotherapeutics: The oncology biotech is laying off around 50% of staff tied to a “strategic refocusing” that will also see the company halt development of its phase 2 immune-stimulating antibody conjugate. The changes reach all the way to the top, with CEO Randall Schatzman and Chief Medical Officer Edith Perez, M.D., being moved to “advisory roles.” Story
May 15 - Trevena: The CNS disorder-focused biotech has implemented a 35% workforce reduction since the end of 2023. The company is also continuing a strategic review of options for Olinvyk, an opioid agonist injection approved for adults with acute pain. Release
May 14 - Bayer: German pharma Bayer is cutting 1,500 jobs across the company. Trickles of layoffs began earlier this year after CEO Bill Anderson looked to slash managerial roles to lessen bureaucracy. Story
May 13 - Takeda: Amid a multi-year reorganization program, Takeda has told employees it will be shuttering a research site in San Diego, a company spokesperson told Fierce Biotech via email. About 340 full-time employees currently work at the San Diego center, though some roles may also be moved to other Takeda research locations, including in Cambridge, Massachusetts. The spokesperson said the pharma is encouraging employees to apply for these positions, which would include relocation support.
Disease of the week
Myasthenia Gravis (MG) is a chronic autoimmune neuromuscular disorder characterized by muscle weakness and fatigue, particularly during physical activity. It primarily affects the voluntary muscles, those that are under conscious control, such as those involved in movement, breathing, swallowing, and speaking.
Causes:
Autoimmune Disorder: Myasthenia Gravis occurs when the immune system mistakenly attacks healthy cells, specifically the receptors for acetylcholine, a neurotransmitter that signals muscle contractions. This results in impaired communication between nerves and muscles, leading to muscle weakness.
Symptoms:
Muscle Weakness: The hallmark symptom of MG is muscle weakness that worsens with activity and improves with rest. This weakness can vary in severity and can affect different muscles in the body.
Drooping Eyelids (Ptosis): Weakness in the muscles that control the eyelids can cause one or both eyelids to droop.
Double Vision (Diplopia): Weakness in the muscles that control eye movement can lead to double vision.
Difficulty Swallowing (Dysphagia): Weakness in the muscles involved in swallowing can cause difficulty swallowing food and liquids.
Difficulty Speaking (Dysarthria): Weakness in the muscles involved in speech production can cause slurred speech.
Fatigue: Muscle fatigue is a common symptom, particularly after sustained use of muscles.
Diagnosis:
Physical Examination: A thorough physical examination, including a neurological examination, is usually the first step in diagnosing MG. The characteristic symptoms, such as muscle weakness and fatigue, are often evident during the examination.
Blood Tests: Blood tests may be conducted to check for the presence of antibodies associated with MG, such as anti-acetylcholine receptor antibodies (AChR) or anti-muscle-specific kinase antibodies (MuSK).
Electrodiagnostic Tests: Electromyography (EMG) and nerve conduction studies may be performed to assess nerve and muscle function.
Edrophonium Test: In this test, a short-acting acetylcholinesterase inhibitor called edrophonium is injected, temporarily improving muscle strength in individuals with MG.
Treatment:
Medications: Medications that enhance the transmission of nerve impulses to muscles, such as acetylcholinesterase inhibitors (e.g., pyridostigmine), are commonly prescribed to improve muscle strength and reduce symptoms.
Immunosuppressive Therapy: Immunosuppressive medications, such as corticosteroids, azathioprine, or mycophenolate mofetil, may be used to suppress the immune system and reduce the production of antibodies that attack healthy cells.
Plasma Exchange (Plasmapheresis): This procedure involves removing antibodies from the blood plasma and replacing it with donor plasma.
Intravenous Immunoglobulin (IVIG) Therapy: IVIG involves administering high doses of antibodies obtained from healthy donors to temporarily modify the immune response.
Thymectomy: Surgical removal of the thymus gland (thymectomy) may be recommended, particularly in individuals with thymoma or those under the age of 60, as the thymus is often abnormal in individuals with MG.
Prognosis:
The prognosis for individuals with Myasthenia Gravis varies depending on the severity of symptoms and the individual's response to treatment. With appropriate management, many individuals with MG can lead relatively normal lives.
In some cases, symptoms may go into remission spontaneously or with treatment. However, periods of exacerbation (flare-ups) may occur, requiring adjustments to treatment.
Lifestyle and Coping:
Conserving Energy: Managing activities to conserve energy and minimize fatigue is important for individuals with MG.
Assistive Devices: The use of assistive devices, such as glasses with prism lenses to correct double vision or eating utensils designed for individuals with hand weakness, can help improve quality of life.
Support Groups: Joining support groups or seeking counseling can provide emotional support and practical tips for coping with the challenges of living with MG.
What I’ve read this week
*Click on the pic to read*