Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
How the market performed this week
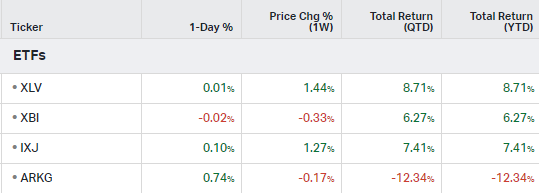
ETFs
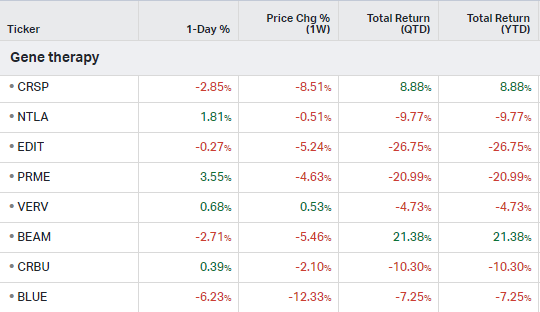
Gene Therapy
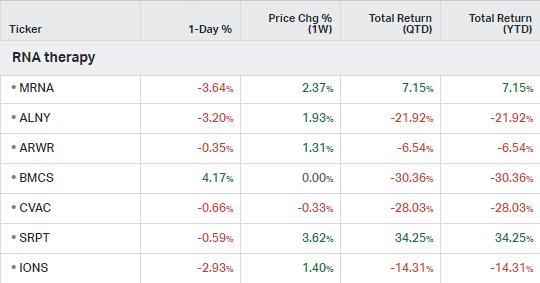
RNA Therapy
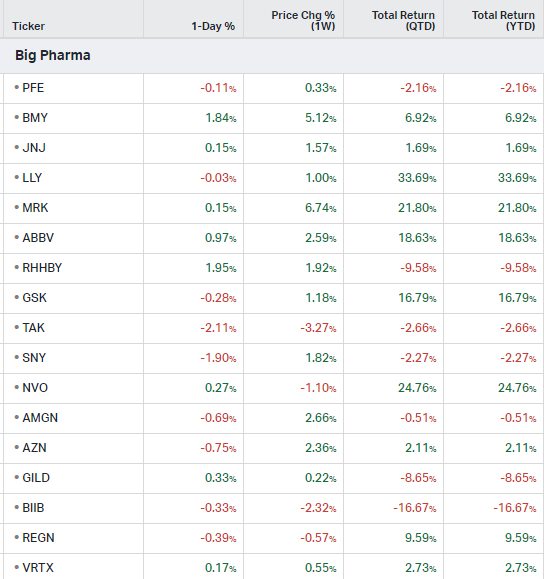
Big Pharma
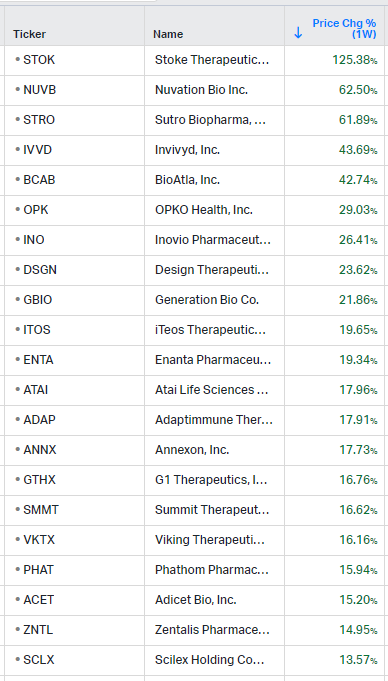
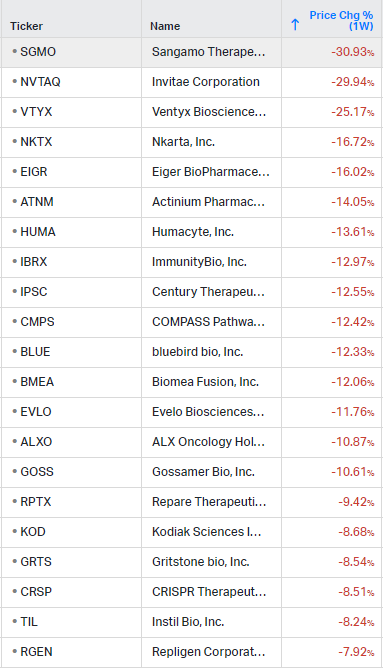
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
SLS
Company Type: Public
Current Phase: I/II
Drug: SLS009
Disease Group: Oncology
Indication: Diffuse Large B-Cell Lymphoma (DLBCL) - NHL
Lead Indication: N
Target: Cyclin Dependent Kinase 9 (CDK-9)
Source Link: PR Newswire
GILD
Company Type: Public
Current Phase: I
Drug: XTX301
Disease Group: Oncology
Indication: Solid Tumors
Lead Indication: N
Target: IL-12 (Interleukin-12) and IL-12 receptor, Immune System
Source Link: BusinessWire
NVS
Company Type: Public
Current Phase: Approved
Drug: Beovu
Disease Group: Ophthalmology
Indication: Diabetic Macular Edema (Ophthalmology)
Lead Indication: N
Target: VEGF (Vascular endothelial growth factor)
Source Link: GlobeNewswire
NVS
Company Type: Public
Current Phase: Approved
Drug: Beovu
Disease Group: Ophthalmology
Indication: Wet Age-Related Macular Degeneration (Wet AMD) (Ophthalmology)
Lead Indication: Y
Target: VEGF (Vascular endothelial growth factor)
Source Link: GlobeNewswire
AVTX
Company Type: Public
Current Phase: I
Drug: AVTX-009
Disease Group: Dermatology
Indication: Hidradenitis Suppurativa
Lead Indication: N
Target: IL-1 (Interleukin-1)
Source Link: GlobeNewswire
ONCO
Company Type: Public
Current Phase: Approved in Europe
Drug: Pepaxto
Disease Group: Oncology
Indication: Multiple Myeloma (MM)
Lead Indication: Y
Target: Angiogenesis, DNA
Source Link: GlobeNewswire
IPSEY
Company Type: Public
Current Phase: Approved
Drug: Onivyde
Disease Group: Oncology
Indication: Pancreatic Cancer
Lead Indication: Y
Target: Topoisomerase I (Topo-I)
Source Link: BusinessWire
288330
Company Type: Public
Current Phase: Preclinical
Drug: BBT-877
Disease Group: Oncology
Indication: Cancer
Lead Indication: N
Target: Autotaxin
Source Link: PR Newswire
VNDA
Company Type: Public
Current Phase: Approved
Drug: Ponvory
Disease Group: Neurology
Indication: Multiple Sclerosis (MS)
Lead Indication: N
Target: Sphingosine 1-Phosphate Receptor (S1P-R)
Source Link: BusinessWire
NVO
Company Type: Public
Current Phase: Preclinical
Drug: FT-3171
Disease Group: Oncology
Indication: Solid Tumors
Lead Indication: Y
Target: Ubiquitin Specific Peptidase (USP1)
Source Link: GlobeNewswire
LLY
Company Type: Public
Current Phase: Approved
Drug: Jaypirca
Disease Group: Oncology
Indication: Mantle Cell Lymphoma - NHL
Lead Indication: Y
Target: Bruton's Tyrosine Kinase (BTK)
Source Link: Nippon Shinyaku
LLY
Company Type: Public
Current Phase: Approved
Drug: Jaypirca
Disease Group: Oncology
Indication: Chronic Lymphocytic Leukemia (CLL)/Small Cell Lymphocytic Lymphoma (SLL) - NHL
Lead Indication: N
Target: Bruton's Tyrosine Kinase (BTK)
Source Link: Nippon Shinyaku
QTTB
Company Type: Public
Current Phase: II
Drug: ADX-914
Disease Group: Allergy
Indication: Atopic Dermatitis (Eczema)
Lead Indication: Y
Target: IL-7 (Interleukin-7) and IL-7 receptor (IL-7R)
Source Link: PR Newswire
SABS
Company Type: Public
Current Phase: II
Drug: SAB-176
Disease Group: Infectious Disease
Indication: Seasonal Influenza Vaccines
Lead Indication: Y
Target: Influenza Virus
Source Link: GlobeNewswire
LABP
Company Type: Public
Current Phase: II
Drug: NX-13
Disease Group: Autoimmune/immunology
Indication: Ulcerative Colitis (UC)
Lead Indication: N
Target: Mitochondria
Source Link: GlobeNewswire
LABP
Company Type: Public
Current Phase: I
Drug: NX-13
Disease Group: Autoimmune/immunology
Indication: Crohn's Disease
Lead Indication: N
Target: Mitochondria
Source Link: GlobeNewswire
LABP
Company Type: Public
Current Phase: Preclinical
Drug: LABP-66
Disease Group: Neurology
Indication: Alzheimer's Disease (AD)
Lead Indication: N
Target: Mitochondria
Source Link: GlobeNewswire
LABP
Company Type: Public
Current Phase: Preclinical
Drug: LABP-66
Disease Group: Neurology
Indication: Multiple Sclerosis (MS)
Lead Indication: Y
Target: Mitochondria
Source Link: GlobeNewswire
LABP
Company Type: Public
Current Phase: Preclinical
Drug: LABP-73
Disease Group: Respiratory
Indication: Asthma
Lead Indication: N
Target: Mitochondria
Source Link: GlobeNewswire
LABP
Company Type: Public
Current Phase: Preclinical
Drug: LABP-69
Disease Group: Autoimmune/immunology
Indication: Rheumatoid Arthritis (RA)
Lead Indication: Y
Target: Plexin Domain Containing 2 (PLXDC2)
Source Link: GlobeNewswire
Clinical trials (LOA=likelihood of approval)
Ironwood Pharmaceuticals, Inc. (IRWD)
Event Type: Trial Data - Top-Line Results
Current Phase: II
Drug: Apraglutide
Disease Group: Autoimmune/immunology
Indication: Graft vs. Host Disease (GVHD) - Treatment
Lead Indication: N
Target: GLP-2 Receptor
LOA: 20%
Source Link: BusinessWire
Lipocine Inc. (LPCN)
Event Type: Trial Data - Updated Results
Current Phase: II
Drug: LPCN 1148
Disease Group: Gastroenterology (Non Inflammatory Bowel Disease)
Indication: Liver Failure / Cirrhosis
Lead Indication: Y
Target: Androgen receptors
LOA: 21%
Source Link: PR Newswire
Aligos Therapeutics, Inc. (ALGS)
Event Type: Trial Data - Updated Results
Current Phase: II
Drug: ALG-055009
Disease Group: Endocrine
Indication: Non-Alcoholic Steatohepatitis (NASH)
Lead Indication: Y
Target: Thyroid hormone receptors (TRs)
LOA: 15%
Source Link: [Not specified]
Bristol Myers Squibb Company (BMY)
Event Type: Trial Data - Top-Line Results
Current Phase: Approved
Drug: Krazati
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Lead Indication: N
Target: KRas
LOA: 100%
Source Link: BusinessWire
Bristol Myers Squibb Company (BMY)
Event Type: Trial Data - Top-Line Results
Current Phase: III
Drug: Zeposia
Disease Group: Autoimmune/immunology
Indication: Crohn's Disease
Lead Indication: N
Target: Sphingosine 1-Phosphate Receptor (S1P-R)
LOA: 61%
Source Link: BusinessWire
TFF Pharmaceuticals, Inc. (TFFP)
Event Type: Trial Data - Updated Results
Current Phase: Development Outside U.S.
Drug: TFF TAC
Disease Group: Autoimmune/immunology
Indication: Lung Transplant Rejection
Lead Indication: N
Target: Calcineurin phosphatase
LOA: Not specified
Source Link: GlobeNewswire
TFF Pharmaceuticals, Inc. (TFFP)
Event Type: Trial Data - Updated Results
Current Phase: II
Drug: TFF VORI
Disease Group: Infectious Disease
Indication: Aspergillosis
Lead Indication: N
Target: Cytochrome p450
LOA: 23%
Source Link: GlobeNewswire
Reviva Pharmaceuticals Holdings, Inc. (RVPH)
Event Type: Trial Data - Updated Results
Current Phase: III
Drug: Brilaroxazine
Disease Group: Psychiatry
Indication: Schizophrenia
Lead Indication: Y
Target: Dopamine 2 (D2) Receptor, Dopamine 3 (D3) Receptor, Dopamine 4 (D4) Receptor, Serotonin 5-HT1A receptor
LOA: 58%
Source Link: ASCPT2024
Moderna, Inc. (MRNA)
Event Type: Trial Data - Top-Line Results
Current Phase: I
Drug: mRNA-1189
Disease Group: Infectious Disease
Indication: Epstein Barr Virus (EBV)
Lead Indication: Y
Target: Epstein Barr Virus (EBV), Immune System
LOA: 13%
Source Link: Moderna
Moderna, Inc. (MRNA)
Event Type: Trial Data - Top-Line Results
Current Phase: I/II
Drug: mRNA-1468
Disease Group: Infectious Disease
Indication: Chickenpox and Shingles - Vaccines and Treatments
Lead Indication: Y
Target: Varicella Zoster Virus (VSV)
LOA: 24%
Source Link: Moderna
Moderna, Inc. (MRNA)
Event Type: Trial Data - Top-Line Results
Current Phase: I
Drug: mRNA-1403
Disease Group: Infectious Disease
Indication: Norovirus
Lead Indication: N
Target: Norovirus
LOA: 13%
Source Link: Moderna
Moderna, Inc. (MRNA)
Event Type: Trial Data - Updated Results
Current Phase: III
Drug: mRNA-1283
Disease Group: Infectious Disease
Indication: COVID-19 Prevention
Lead Indication: Y
Target: SARS-CoV-2
LOA: 59%
Source Link: Moderna
Moderna, Inc. (MRNA)
Event Type: Trial Data - Updated Results
Current Phase: BLA
Drug: mRNA-1345
Disease Group: Infectious Disease
Indication: Respiratory Syncytial Virus (RSV) Prevention
Lead Indication: Y
Target: Immune System, RSV, Respiratory Syncytial Virus
LOA: 99%
Source Link: Moderna
Moderna, Inc. (MRNA)
Event Type: Trial Data - Top-Line Results
Current Phase: BLA
Drug: mRNA-1345
Disease Group: Infectious Disease
Indication: Respiratory Syncytial Virus (RSV) Prevention
Lead Indication: Y
Target: Immune System, RSV, Respiratory Syncytial Virus
LOA: 99%
Source Link: Moderna
Sagimet Biosciences (SGMT)
Event Type: Trial Announcement - Trial Completed
Current Phase: II
Drug: Denifanstat
Disease Group: Endocrine
Indication: Non-Alcoholic Steatohepatitis (NASH)
Lead Indication: Y
Target: Fatty acids
LOA: 26%
Source Link: GlobeNewswire
Akebia Therapeutics, Inc. (AKBA)
Event Type: Regulatory - Approval (U.S.)
Current Phase: Approved
Drug: Vafseo
Disease Group: Hematology
Indication: Anemia Due to Chronic Kidney Disease, Dialysis-Dependent
Lead Indication: N
Target: Hypoxia-Inducible Factor-Prolyl Hydroxylase (HIF-PH)
LOA: 100%
Source Link: PR Newswire
Lixte Biotechnology Holdings, Inc. (LIXT)
Event Type: Trial Data - Preclinical Results
Current Phase: Preclinical
Drug: LB-100
Disease Group: Oncology
Indication: Colorectal Cancer (CRC)
Lead Indication: N
Target: PP2A (Serine/threonine-specific protein phosphatase 2A)
LOA: Not specified
Source Link: GlobeNewswire
Heidelberg Pharma AG (WL6)
Event Type: Regulatory - Orphan Drug Designation (U.S.)
Current Phase: I/II
Drug: HDP-101
Disease Group: Oncology
Indication: Multiple Myeloma (MM)
Lead Indication: Y
Target: B-cell maturation antigen (BCMA), RNA polymerase
LOA: 11%
Source Link: Heidelberg Pharma AG
Adaptimmune Therapeutics plc (ADAP)
Event Type: Trial Data - Published Results
Current Phase: II
Drug: Afami-cel
Disease Group: Oncology
Indication: Liposarcoma
Lead Indication: N
Target: Cluster of Differentiation 107a (CD107a) / LAMP-1, Melanoma antigen-encoding gene (MAGE), Stem Cells/Other Cell Therapies, T-Cell Receptor (TCR)
LOA: 13%
Source Link: Newsfile Corp
AbbVie Inc. (ABBV)
Event Type: Trial Data - Published Results
Current Phase: II/III
Drug: ABBV-RGX-314
Disease Group: Ophthalmology
Indication: Wet Age-Related Macular Degeneration (Wet AMD) (Ophthalmology)
Lead Indication: N
Target: VEGF (Vascular endothelial growth factor)
LOA: 55%
Source Link: PR Newswire
Moderna, Inc. (MRNA)
Event Type: Trial Data - Updated Results
Current Phase: III
Drug: mRNA-1010
Disease Group: Infectious Disease
Indication: Seasonal Influenza Vaccines
Lead Indication: Y
Target: Immune System, Influenza Virus
LOA: 61%
Source Link: Moderna
Moderna, Inc. (MRNA)
Event Type: Trial Data - Top-Line Results
Current Phase: III
Drug: mRNA-1283
Disease Group: Infectious Disease
Indication: COVID-19 Prevention
Lead Indication: Y
Target: SARS-CoV-2
LOA: 59%
Source Link: AccessWire
SELLAS Life Sciences Group, Inc. (SLS)
Event Type: Trial Data - Updated Results
Current Phase: I/II
Drug: SLS009
Disease Group: Oncology
Indication: Acute Myelogenous Leukemia (AML)
Lead Indication: N
Target: Cyclin Dependent Kinase 9 (CDK-9)
LOA: 11%
Source Link: GlobeNewswire
Praxis Precision Medicines, Inc. (PRAX)
Event Type: Trial Data - Top-Line Results
Current Phase: II
Drug: PRAX-628
Disease Group: Neurology
Indication: Seizure Disorders (Epilepsy)
Lead Indication: N
Target: Sodium Channels
LOA: 13%
Source Link: GlobeNewswire
Zevra Therapeutics, Inc. (ZVRA)
Event Type: Trial Data - Updated Results
Current Phase: II
Drug: KP1077
Disease Group: Neurology
Indication: Narcolepsy
Lead Indication: Y
Target: Dopamine Reuptake, Norepinephrine (Noradrenaline) Reuptake
LOA: 12%
Source Link: GlobeNewswire
Viking Therapeutics, Inc. (VKTX)
Event Type: Trial Data - Updated Results
Current Phase: II
Drug: VK2735
Disease Group: Metabolic
Indication: Obesity
Lead Indication: N
Target: GIP Receptor (GIPR)/Glucose-Dependent Insulinotropic Polypeptide Receptor, GLP-1 Receptor
LOA: 28%
Source Link: PR Newswire
Merck & Co., Inc. (MRK)
Event Type: Regulatory - Approval (U.S.)
Current Phase: Approved
Drug: WINREVAIR
Disease Group: Cardiovascular
Indication: Pulmonary Arterial Hypertension (PAH) and Pulmonary Hypertension (PH)
Lead Indication: Y
Target: Activin A, Activin Receptor Type-2A (ACVR2A) (ACTRIIA), Transforming Growth Factor-beta (TGF-beta) and Superfamily
LOA: 100%
Source Link: BusinessWire
Defence Therapeutics Inc. (DTC)
Event Type: Trial Data - Preclinical Results
Current Phase: Preclinical
Drug: ARM-002TM
Disease Group: Oncology
Indication: Melanoma
Lead Indication: N
Target: Unknown
LOA: Not specified
Source Link: Defence Therapeutics
MEI Pharma, Inc. (MEIP)
Event Type: Trial Data - Updated Results
Current Phase: I
Drug: Voruciclib
Disease Group: Oncology
Indication: Acute Myelogenous Leukemia (AML)
Lead Indication: N
Target: Cyclin Dependent Kinase 4 (CDK-4), Cyclin Dependent Kinase 9 (CDK-9)
LOA: 5%
Source Link: BusinessWire
Axsome Therapeutics, Inc. (AXSM)
Event Type: Trial Data - Top-Line Results
Current Phase: III
Drug: AXS-12
Disease Group: Neurology
Indication: Narcolepsy
Lead Indication: Y
Target: Norepinephrine (Noradrenaline) Reuptake
LOA: 57%
Source Link: GlobeNewswire
Astria Therapeutics, Inc. (ATXS)
Event Type: Trial Data - Top-Line Results
Current Phase: II
Drug: STAR-0215
Disease Group: Autoimmune/immunology
Indication: Hereditary Angioedema (HAE)
Lead Indication: Y
Target: Kinin-Kallikrein System
LOA: 19%
Source Link: BusinessWire
Matinas BioPharma Holdings, Inc. (MTNB)
Event Type: Trial Data - Preclinical Results
Current Phase: Preclinical
Drug: LNC Docetaxel
Disease Group: Oncology
Indication: Melanoma
Lead Indication: N
Target: Unknown
LOA: Not specified
Source Link: GlobeNewswire
Stoke Therapeutics, Inc. (STOK)
Event Type: Trial Data - Updated Results
Current Phase: I/II
Drug: STK-001
Disease Group: Neurology
Indication: Dravet Syndrome (Epilepsy)
Lead Indication: Y
Target: Sodium Channel Protein SCN1A
LOA: 15%
Source Link: BusinessWire
NKGen Biotech, Inc. (NKGNW)
Event Type: Trial Data - Updated Results
Current Phase: I/II
Drug: SNK01
Disease Group: Neurology
Indication: Alzheimer's Disease (AD)
Lead Indication: Y
Target: Immune System, Natural Killer Cells (NK Cells)
LOA: 12%
Source Link: GlobeNewswire
Moleculin Biotech, Inc. (MBRX)
Event Type: Trial Data - Updated Results
Current Phase: I/II
Drug: Annamycin
Disease Group: Oncology
Indication: Acute Myelogenous Leukemia (AML)
Lead Indication: Y
Target: Topoisomerase II (DNA gyrase)
LOA: 11%
Source Link: PR Newswire
Marvel Biosciences Corp. (MRVL)
Event Type: Trial Data - Preclinical Results
Current Phase: Preclinical
Drug: MB-204
Disease Group: Neurology
Indication: Alzheimer's Disease (AD)
Lead Indication: Y
Target: Adenosine A2a Receptor
LOA: Not specified
Source Link: Newsfile Corp
Financing events
Aeovian Pharmaceuticals:
Description: Develops innovative therapeutics for rare and age-related diseases.
Vertical: Life Sciences
Deal Date: March 28, 2024
Deal Type: Later Stage VC
Investors: Hevolution Foundation (lead), Evotec, b2venture, Apollo Health Ventures, venBio, Sofinnova Investments
Deal Size: $50 million
Andson Biotech:
Description: Develops novel Process Analytical Technology (PAT) for cell-based bioprocesses.
Verticals: HealthTech, Life Sciences
Deal Date: March 26, 2024
Deal Type: Early Stage VC
Investors: Undisclosed
Deal Size: $3.6 million
AnHeart Therapeutics:
Description: Operates a biopharmaceutical company focusing on improving cancer patients' lives.
Verticals: Life Sciences, Oncology
Deal Date: March 25, 2024
Deal Type: Merger/Acquisition
Acquirer: Nuvation Bio (NYS: NUVB)
Avalo Therapeutics (NAS: AVTX):
Description: Clinical-stage precision medicine company developing targeted therapeutics.
Verticals: Life Sciences, Oncology
Deal Date: March 27, 2024
Deal Type: PIPE
Investors: Petrichor Partners, OrbiMed, Deep Track Capital, Commodore Capital, RA Capital Management, TCG Crossover Management, BVF Partners
Deal Size: $185 million
Avenzo:
Description: Operates a clinical-stage oncology platform focusing on novel cancer treatments.
Verticals: HealthTech, Life Sciences, Oncology
Deal Date: March 26 & 27, 2024
Deal Type: Series A & Series A1 VC
Investors: Deep Track Capital, Sands Capital, Sofinnova Investments, and others
Deal Size: $223.35 million (Series A), $150 million (Series A1)
BioXcel Therapeutics (NAS: BTAI):
Description: Clinical-stage biopharmaceutical company using AI to identify new medicines.
Verticals: Artificial Intelligence & Machine Learning, Life Sciences, Oncology
Deal Date: March 25, 2024
Deal Type: PIPE
Investors: Undisclosed
Deal Size: $8.86 million
Boundless Bio (NAS: BOLD):
Description: Clinical-stage oncology company focusing on extrachromosomal DNA.
Verticals: Life Sciences, Oncology
Deal Date: March 28, 2024
Deal Type: IPO
Deal Size: $100 million
Century Health:
Description: Develops healthcare technology applying AI to clinical data.
Verticals: Artificial Intelligence & Machine Learning, HealthTech, Life Sciences
Deal Date: March 27, 2024
Deal Type: Seed Round
Investors: 2048 Ventures (lead), Everywhere, Alumni Ventures, LifeX Ventures, Travis May, Christine Lemke
Deal Size: $2 million
CervoMed (NAS: CRVO):
Description: Develops drugs for central nervous system diseases, including Alzheimer's.
Verticals: Life Sciences
Deal Date: March 28, 2024
Deal Type: PIPE
Investors: Soleus Capital, RA Capital Management, Armistice Capital, Special Situations Funds
Deal Size: $149.4 million
CND Life Sciences:
Description: Develops a medical diagnostic platform for neurodegenerative diseases.
Verticals: Digital Health, HealthTech, Life Sciences
Deal Date: March 27, 2024
Deal Type: Later Stage VC
Investors: Undisclosed
Deal Size: $3.46 million
Reduction in force (RIF)
March 28 - Omega Therapeutics: The mRNA-focused company is laying off 35% of its workforce as part of a "cost reduction and strategic prioritization initiative." Omega will also focus resources on certain preclinical programs including OTX-2101 for non-small cell lung cancer, its HNF4A program in liver regeneration, and an epigenomic controller for obesity in collaboration with Novo Nordisk. Release
March 28 - Xilio: The biotech may have secured $43.5 million from Gilead for the license to a tumor-activated IL-12, but Xilio also let go of 15 employees on the same day. The decision to reduce its workforce by 21% was a response to the biotech's narrowing focus, having handed over one IL-12 drug to Gilead and halting monotherapy development of another. Story
March 27 - AlmataBio: Avalo Therapeutics agreed to hand over $22 million in stock and cash to acquire AlmataBio, but Avalo only has eyes for Almata's anti-IL-1β monoclonal antibody AVTX-009. As a result, none of Almata's employees will be keeping their job under the new owner. Story
March 27 - Bayer: The pharma will lay off 90 employees working at the company's U.S. headquarters in Whippany, New Jersey. The cuts, which are effective on June 19, were revealed in a New Jersey WARN notice and confirmed by Bayer over email. Story
March 26 - Bristol Myers Squibb: Two months after closing its multibillion-dollar acquisition of Mirati Therapeutics, BMS is bidding adieu to 252 workers at the biotech's headquarters in California. Story
Disease of the week
Nodding Syndrome is a rare neurological disorder primarily affecting children in specific regions of Sub-Saharan Africa, particularly South Sudan, Uganda, and Tanzania. It's characterized by repetitive nodding movements, typically accompanied by cognitive impairment, stunted growth, and seizures. Here are some key points about Nodding Syndrome:
Epidemiology: Nodding Syndrome has been reported primarily in regions affected by conflict, displacement, and poverty. It's most prevalent in rural areas with limited access to healthcare and sanitation facilities.
Symptoms: The hallmark symptom of Nodding Syndrome is repetitive nodding of the head, often triggered by cold weather, food consumption, or other stimuli. Other symptoms may include seizures (typically generalized tonic-clonic seizures), cognitive impairment, stunted growth, malnutrition, and behavioral problems.
Onset and Progression: Nodding Syndrome typically begins between the ages of 5 and 15 years old. The nodding episodes tend to worsen over time, and the condition can lead to severe disability, including the inability to walk or talk.
Potential Causes: The exact cause of Nodding Syndrome is not fully understood, but it's believed to be multifactorial, involving genetic, environmental, and infectious factors. There is some evidence suggesting a possible association with parasitic infections, particularly Onchocerca volvulus, the parasite responsible for river blindness, although the link is not definitive.
Diagnosis: Diagnosis of Nodding Syndrome is primarily based on clinical presentation, including the characteristic nodding episodes and other associated symptoms. Medical history, physical examination, and neurological assessments are typically conducted to rule out other conditions with similar symptoms.
Treatment: Currently, there is no specific cure for Nodding Syndrome. Treatment mainly focuses on managing symptoms and improving the quality of life for affected individuals. This may involve antiepileptic medications to control seizures, nutritional support to address malnutrition, and interventions to address cognitive and developmental challenges.
Prevention and Research: Efforts to prevent Nodding Syndrome involve addressing underlying risk factors such as poverty, malnutrition, and infectious diseases. Research into the underlying causes and potential treatments for the condition is ongoing, but more resources and attention are needed to better understand and combat this devastating disorder.
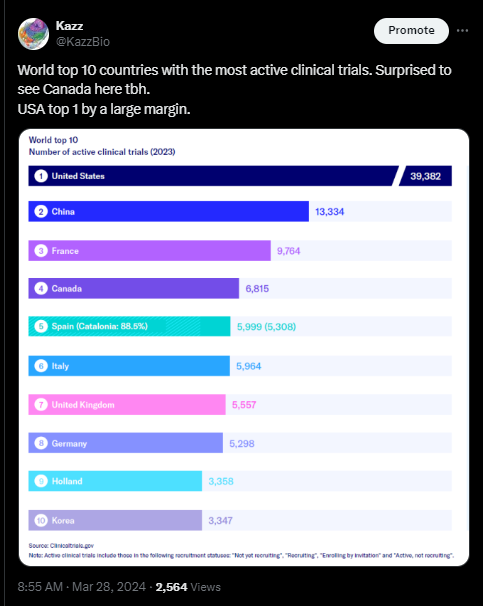
What I’ve read this week
*Click on the pic to read*