Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
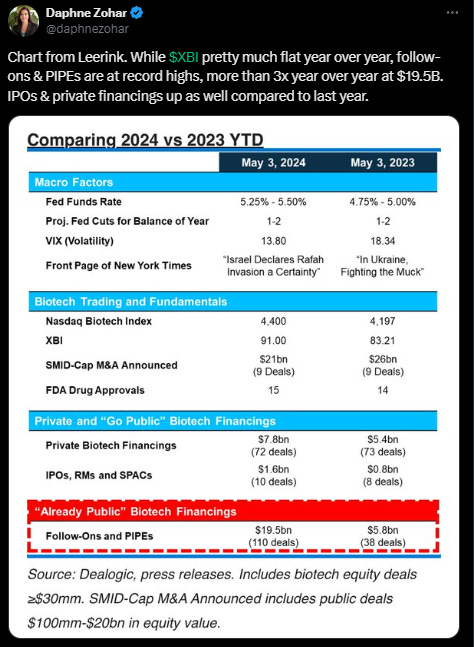
Market
Notes from analysts
Venture Capital Market
M&A
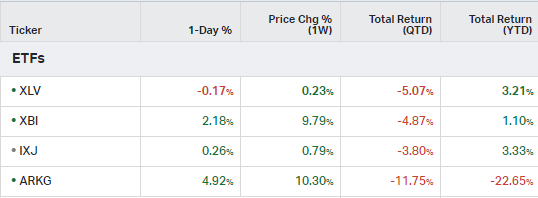
How the market performed this week
ETFs
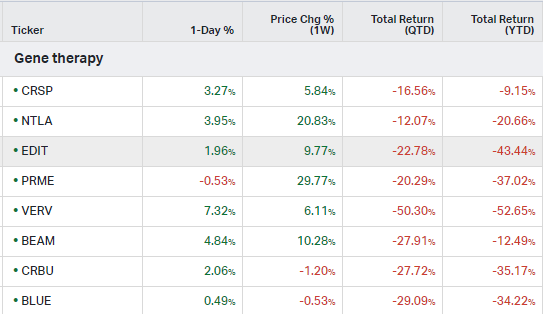
Gene Therapy
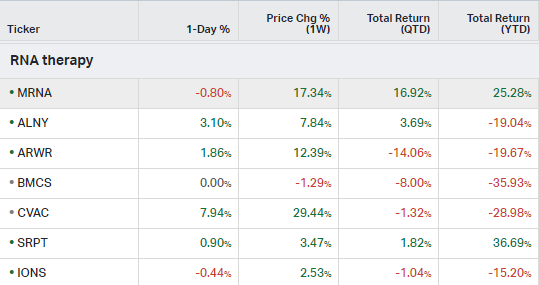
RNA Therapy
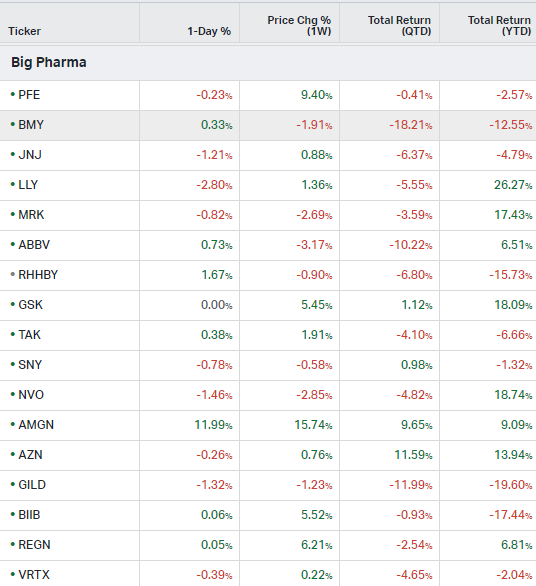
Big Pharma
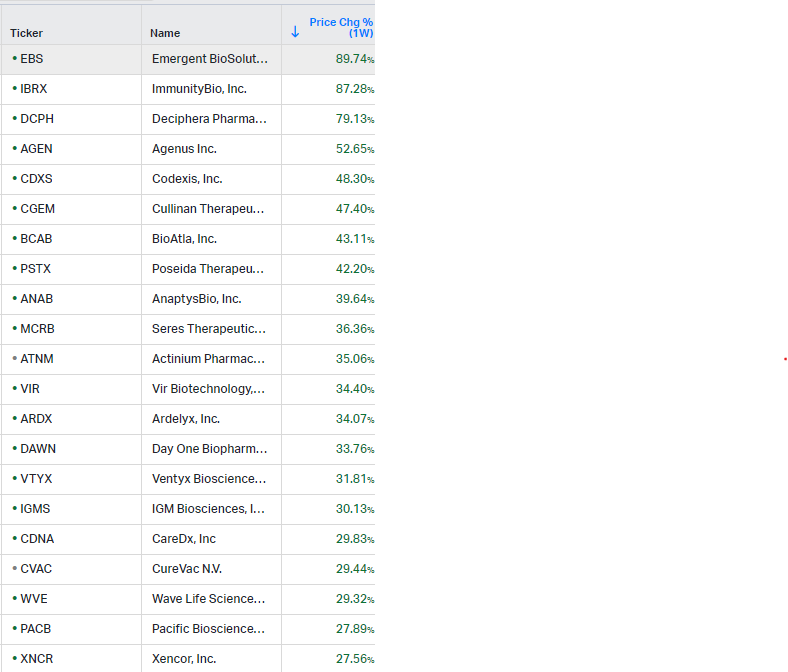
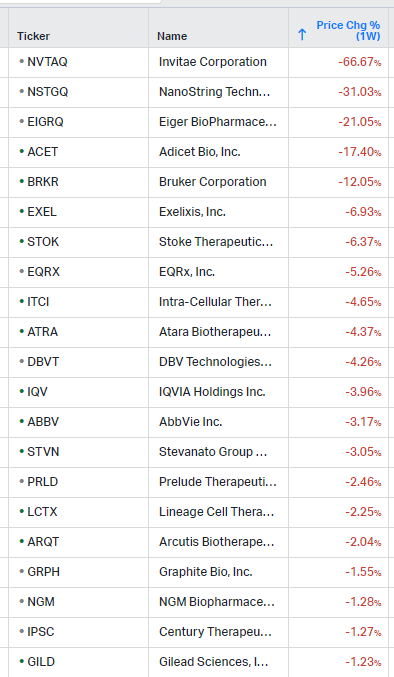
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
ASLAN Pharmaceuticals Pte Ltd (Symbol: ASLN)
Current Phase: IIb
Drug: Eblasakimab
Disease Group: Allergy
Indication: Atopic Dermatitis (Eczema)
Target: IL-13 (Interleukin-13), IL-4 Receptor (IL-4R)
LOA: 20%
Partner Companies: CSL Limited, Merck & Co., Inc., Nippon Zenyaku Kogyo Co Ltd
Source Link: ASLAN Pharmaceuticals
Crossject (Symbol: ALCJ)
Current Phase: Preclinical
Drug: Zepizure
Disease Group: Neurology
Indication: Seizure Disorders (Epilepsy)
Target: GABA-A Receptor
Partner Companies: AFT Pharmaceuticals Limited, Desitin Pharmaceuticals GmbH
Source Link: Crossject Update
Amgen, Inc. (Symbol: AMGN)
Current Phase: III
Drug: AMG 890
Disease Group: Cardiovascular
Indication: Dyslipidemia / Hypercholesterolemia
Target: Lipoprotein (a) [Lp(a)]
LOA: 47%
Partner Companies: Arrowhead Pharmaceuticals, Inc., Royalty Pharma plc
Source Link: Amgen Update
Harmony Biosciences Holdings, Inc. (Symbol: HRMY)
Current Phase: Various (I, II, IND)
Drugs: EPX-100, EPX-200, EPX-300 (various indications for epilepsy and related disorders)
Source Link: Harmony Biosciences
Alnylam Pharmaceuticals Inc. (Symbol: ALNY)
Current Phase: Approved
Drugs: Amvuttra, Givlaari, Onpattro, Oxlumo (various metabolic and renal diseases)
Partner Companies: Medison Pharma Ltd., Sanofi, Arbutus Biopharma Corporation, GENESIS Pharma S.A., Ionis Pharmaceuticals, Inc., taiba-ME, Novo Nordisk A/S, Royalty Pharma plc
Source Link: Alnylam Pharmaceuticals
Novartis AG (Symbol: NVS)
Current Phase: Approved
Drug: Biosimilar Denosumab (Sandoz)
Disease Group: Endocrine
Indication: Osteoporosis / Osteopenia
Target: RANK Ligand (RANKL)
Partner Companies: Sandoz AG
Source Link: Novartis Update
Deciphera Pharmaceuticals, Inc. (Symbol: DCPH)
Current Phase: Various (Approved, Preclinical, Phase I/II, III)
Drugs: Qinlock, Vimseltinib, DCC-3116, DCC-3084, DCC-3009 (oncology and autoimmune diseases)
Partner Companies: GENESIS Pharma S.A., Medison Pharma Ltd., Specialised Therapeutics, Zai Lab Ltd., and others
Source Link: Deciphera Pharmaceuticals
X4 Pharmaceuticals, Inc. (Symbol: XFOR)
Current Phase: Approved
Drug: Xolremdi
Disease Group: Autoimmune/immunology
Indication: Primary Immunodeficiencies
Target: Chemokine (C-X-C motif) Receptor 4 (CXCR4), Immune System
Partner Companies: Abbisko Therapeutics Co., Ltd.
Source Link: X4 Pharmaceuticals
Clinical trials (LOA=likelihood of approval)
Kancera AB (Symbol: KAN)
Event Type: Trial Data - Top-Line Results
Current Phase: Development Outside U.S.
Drug: KAND145
Disease Group: Oncology
Indication: Ovarian Cancer
Target: CX3C chemokine receptor 1 (CX3CR1), Immune System
LOA: N/A
Source Link: Kancera Report
AEON Biopharma, Inc. (Symbol: AEON)
Event Type: Trial Data - Updated Results
Current Phase: II
Drug: ABP-450
Disease Group: Neurology
Indication: Migraine and Other Headaches
Target: Botulinum toxin, SNARE Proteins
LOA: 10%
Source Link: AEON Biopharma Report
Gossamer Bio, Inc. (Symbol: GOSS)
Event Type: Trial Data - Published Results
Current Phase: III
Drug: GB002
Disease Group: Cardiovascular
Indication: Pulmonary Arterial Hypertension (PAH) and Pulmonary Hypertension (PH)
Target: Platelet-derived growth factor (PDGF)
LOA: 49%
Source Link: Gossamer Bio Report
Organogenesis Inc. (Symbol: ORGO)
Event Type: Trial Data - Top-Line Results
Current Phase: III
Drug: ReNu
Disease Group: Rheumatology (Non Autoimmune)
Indication: Osteoarthritis
Target: Unknown
LOA: 38%
Source Link: Organogenesis Report
Allarity Therapeutics A/S (Symbol: ALLR)
Event Type: Trial Announcement - Trial Completed
Current Phase: II
Drug: Stenoparib
Disease Group: Oncology
Indication: Ovarian Cancer
Target: Poly ADP-Ribose Polymerase (PARP)
LOA: 11%
Source Link: Allarity Therapeutics Report
Amgen, Inc. (Symbol: AMGN)
Event Type: Trial Announcement - Trial Completed
Current Phase: I
Drug: AMG-786
Disease Group: Metabolic
Indication: Obesity
Target: Unknown
LOA: 15%
Source Link: Amgen Report
Kodiak Sciences Inc. (Symbol: KOD)
Event Type: Trial Data - Updated Results
Current Phase: III
Drug: KSI-301
Disease Group: Ophthalmology
Indication: Diabetic Retinopathy
Target: VEGF (Vascular endothelial growth factor)
LOA: 54%
Source Link: Kodiak Sciences Report
Kodiak Sciences Inc. (Symbol: KOD) - Another Drug
Event Type: Trial Data - Preclinical Results
Current Phase: I
Drug: KSI-501
Disease Group: Ophthalmology
Indication: Other Ophthalmological Indications
Target: IL-6 (Interleukin-6), VEGF (Vascular endothelial growth factor)
LOA: 21%
Source Link: Kodiak Sciences Additional Report
AstraZeneca PLC (Symbol: AZN)
Event Type: Trial Data - Top-Line Results
Current Phase: Approved
Drug: Calquence
Disease Group: Oncology
Indication: Mantle Cell Lymphoma - NHL
Target: Bruton's Tyrosine Kinase (BTK)
LOA: 100%
Source Link: AstraZeneca Report
Defence Therapeutics Inc. (Symbol: DTC)
Event Type: Trial Data - Preclinical Results
Current Phase: Preclinical
Drug: ARM-002TM
Disease Group: Oncology
Indication: Pancreatic Cancer
Target: Unknown
LOA: N/A
Source Link: Defence Therapeutics Report
Aileron Therapeutics, Inc. (Symbol: ALRN)
Event Type: Trial Data - Top-Line Results
Current Phase: I
Drug: LTI-03
Disease Group: Respiratory
Indication: Idiopathic Pulmonary Fibrosis (IPF)
Target: Unknown
LOA: 7%
Source Link: Aileron Therapeutics Report
Palisade Bio, Inc. (Symbol: PALI)
Event Type: Trial Data - Preclinical Results
Current Phase: Preclinical
Drug: PALI-2108
Disease Group: Autoimmune/immunology
Indication: Ulcerative Colitis (UC)
Target: Phosphodiesterase 4 (PDE4)
LOA: N/A
Source Link: Palisade Bio Report
SELLAS Life Sciences Group, Inc. (Symbol: SLS)
Event Type: Trial Data - Updated Results
Current Phase: I/II
Drug: SLS009
Disease Group: Oncology
Indication: Acute Myelogenous Leukemia (AML)
Target: Cyclin Dependent Kinase 9 (CDK-9)
LOA: 11%
Source Link: SELLAS Life Sciences Report
Merck & Co., Inc. (Symbol: MRK)
Event Type: Trial Data - Final Results
Current Phase: Approved
Drug: Keytruda
Disease Group: Oncology
Indication: Gastric Cancer
Target: Immune System, Programmed death-1 receptor (PD-1)
LOA: 100%
Source Link: Merck & Co. Report
Belite Bio, Inc. (Symbol: BLTE)
Event Type: Trial Data - Updated Results
Current Phase: III
Drug: LBS-008
Disease Group: Ophthalmology
Indication: Stargardt Disease
Target: Retinol binding protein 4 (RBP4)
LOA: 51%
Source Link: Belite Bio Update
Acurx Pharmaceuticals, Inc. (Symbol: ACXP)
Event Type: Trial Data - Updated Results
Current Phase: II
Drug: Ibezapolstat
Disease Group: Infectious Disease
Indication: Clostridium difficile-Associated Diarrhea/Infection (CDAD/CDI)
Target: DNA polymerase
LOA: 23%
Source Link: Acurx Pharmaceuticals Update
Basilea Pharmaceutica Ltd. (Symbol: BSLN)
Event Type: Trial Data - Updated Results
Current Phase: Approved
Drug: Zeftera
Disease Group: Infectious Disease
Indication: Septicemia or Bacteremia (including Endocarditis)
Target: Penicillin Binding Proteins (PBP)
LOA: 100%
Source Link: Basilea Pharmaceutica Update
Newron Pharmaceuticals S.p.A. (Symbol: NWRN)
Event Type: Trial Data - Updated Results
Current Phase: II/III
Drug: Evenamide
Disease Group: Psychiatry
Indication: Schizophrenia
Target: Sodium Channels
LOA: 50%
Source Link: Newron Pharmaceuticals Update
Aurinia Pharmaceuticals Inc. (Symbol: AUPH)
Event Type: Regulatory - Change to Product Label (U.S.)
Current Phase: Approved
Drug: Lupkynis
Disease Group: Autoimmune/immunology
Indication: Lupus Nephritis
Target: Calcineurin phosphatase
LOA: 100%
Source Link: Aurinia Pharmaceuticals Update
Vaxart, Inc. (Symbol: VXRT)
Event Type: Trial Data - Top-Line Results
Current Phase: II
Drug: Bivalent Norovirus Vaccine (Vaxart)
Disease Group: Infectious Disease
Indication: Norovirus
Target: Immune System, Norovirus
LOA: 25%
Source Link: Vaxart Update
Gritstone bio, Inc. (Symbol: GRTS)
Event Type: Trial Data - Updated Results
Current Phase: I
Drug: CORAL
Disease Group: Infectious Disease
Indication: COVID-19 Prevention
Target: SARS-CoV-2
LOA: 13%
Source Link: Gritstone bio Update
Virpax Pharmaceuticals, Inc. (Symbol: VRPX)
Event Type: Trial Data - Preclinical Results
Current Phase: Preclinical
Drug: Probudur
Disease Group: Neurology
Indication: Postsurgical Pain
Target: Sodium Channels
LOA: N/A
Source Link: Virpax Pharmaceuticals Update
CERo Therapeutics Holdings, Inc (Symbol: CERO)
Event Type: Trial Data - Preclinical Results
Current Phase: Preclinical
Drug: CER-1236
Disease Group: Oncology
Indication: Hematologic Cancer
Target: Immune System, Stem Cells/Other Cell Therapies
LOA: N/A
Source Link: CERo Therapeutics Update
Financing events
Cyclacel Pharmaceuticals (NAS: CYCC)
Description: Clinical-stage biopharmaceutical company developing medicines for cancer and proliferative diseases.
Verticals: Life Sciences, Oncology
Deal Date: May 2, 2024
Deal Type: PIPE
Deal Synopsis: Received $8 million from undisclosed investors through private placement.
Investors: Undisclosed
Deal Size: $8.00 million
Deciphera Pharmaceuticals (NAS: DCPH)
Description: Develops kinase-inhibiting drugs for cancers and immunological diseases.
Verticals: Life Sciences, Oncology
Deal Date: April 29, 2024
Deal Type: Merger/Acquisition
Deal Synopsis: To be acquired by ONO Pharmaceutical for $2.4 billion.
Investors: ONO Pharmaceutical (TKS: 4528) (Gyo Sagara)
Deal Size: $2.4 billion
Enlaza Therapeutics
Description: Develops novel protein therapeutics leveraging synthetic biology.
Verticals: HealthTech, Life Sciences
Deal Date: April 30, 2024
Deal Type: Early Stage VC
Deal Synopsis: Raised $100 million in Series A funding.
Investors: JPMorgan Life Sciences Private Capital, Amgen Ventures, Regeneron Ventures, others
Deal Size: $100 million
EVQLV
Description: Develops antibody design platforms for therapeutic antibodies.
Verticals: Artificial Intelligence & Machine Learning, HealthTech, Life Sciences
Deal Date: April 30, 2024
Deal Type: Early Stage VC
Deal Synopsis: Raised $520,000 from Medina Ventures and BIP Ventures.
Investors: BIP Ventures, Medina Ventures
Deal Size: $0.52 million
HelixNano
Description: Uses AI in synthetic biology for gene therapies and genome engineering.
Verticals: Artificial Intelligence & Machine Learning, Life Sciences, Nanotechnology
Deal Date: May 1, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised an undisclosed amount from LongeVC.
Investors: LongeVC
Laminar Pharma
Description: Focuses on the discovery and development of MLT-based drugs.
Verticals: HealthTech, Life Sciences, LOHAS & Wellness
Deal Date: April 30, 2024
Deal Type: Later Stage VC
Deal Synopsis: Closed on EUR 7 million in funding for drug trials.
Investors: Dentol Capital
Deal Size: €7.51 million
Locate Bio
Description: Develops regenerative medicine platform for advanced delivery systems.
Verticals: Life Sciences
Deal Date: April 30, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised GBP 9.2 million for clinical studies.
Investors: BGF, Mercia Asset Management
Deal Size: £11.51 million
Mariana Oncology
Description: Focuses on targeted radiopharmaceuticals for cancer treatment.
Verticals: Life Sciences, Oncology
Deal Date: May 2, 2024
Deal Type: Merger/Acquisition
Deal Synopsis: Acquired by Novartis.
Investors: Novartis (SWX: NOVN)
Medicenna Therapeutics (TSE: MDNA)
Description: Develops immuno-oncology therapies, focusing on IL-2, IL-4, and IL-13 Superkines.
Verticals: Life Sciences, Oncology
Deal Date: April 30, 2024
Deal Type: PIPE
Deal Synopsis: Received CAD 20 million for the development of cancer therapies.
Investors: RA Capital Management
Deal Size: CAD 20 million
Mustang Bio (NAS: MBIO)
Description: Focuses on cell and gene therapies for cancers and rare genetic diseases.
Verticals: Life Sciences, Oncology
Deal Date: May 1, 2024
Deal Type: Public Investment 2nd Offering
Deal Synopsis: Second public offering, sold 1,160,000 shares.
Investors: Not specified
Deal Size: $0.27 million
PlaqueTec
Description: Develops biomarker systems for drug development targeting coronary artery disease.
Verticals: Life Sciences
Deal Date: April 30, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised GBP 6.37 million to support BIOPATTERN trial.
Investors: Lord Moynihan, Future Fund (UK), others
Deal Size: £7.97 million
Renovo Concepts
Description: Develops devices for treating traumatic brain, heart, and spine injuries.
Verticals: HealthTech, Life Sciences
Deal Date: April 30, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $1.39 million for development of therapeutic devices.
Investors: Undisclosed
Deal Size: $1.39 million
TFF Pharmaceuticals (NAS: TFFP)
Description: Develops inhaled dry powder drugs based on Thin Film Freezing technology.
Verticals: Life Sciences
Deal Date: May 1, 2024
Deal Type: Public Investment 2nd Offering
Deal Synopsis: Raised $1.66 million in second public offering.
Investors: Not specified
Deal Size: $1.66 million
Transcripta Bio
Description: Uses AI for drug-gene discovery and development.
Verticals: Life Sciences
Deal Date: April 29, 2024
Deal Type: Later Stage VC
Deal Synopsis: Raised $10 million for expansion and scaling.
Investors: BlueYard Capital, JAZZ Venture Partners
Deal Size: $10 million
Vectiopep
Description: Develops cancer immunotherapies focusing on RNA delivery and peptide-based technologies.
Verticals: Life Sciences, Oncology
Deal Date: May 2, 2024
Deal Type: Early Stage VC
Deal Synopsis: Raised an undisclosed amount for research and development.
Investors: Ivo Remmelg, Dag Nurm, UniTartu Ventures
Deal Size: Undisclosed
Vincerx Pharma (NAS: VINC)
Description: Focuses on developing new therapies for cancer.
Verticals: Life Sciences, Oncology
Deal Date: January 19, 2022
Deal Type: Public Investment 2nd Offering
Deal Synopsis: Raised $4.5 million in second public offering.
Investors: Not specified
Deal Size: $4.5 million
Reduction in force (RIF)
May 2 - Chroma Medicine: The Boston-based biotech working to develop epigenetic medicines has laid off an undisclosed number of staffers. Roles affected included some general & administrative and R&D positions, a company spokesperson told Fierce via email. The preclinical company is now focused on advancing some of its programs to the clinic, the spokesperson said.
May 1 - Emergent BioSolutions: About two months into his CEO job at the CDMO-turned-biopharma company, Joseph Papa has laid out his turnaround plan that includes reducing the current workforce by about 300 employees. The company is also shuttering its Baltimore-Bayview drug substance manufacturing facility and its Rockville drug product facility, both of which are located in Maryland. Story
Disease of the week
Stiff Person Syndrome (SPS) is a rare, progressive neurological disorder characterized by significant rigidity and stiffness, primarily affecting the axial muscles (those of the trunk and torso). The condition can lead to severe muscle spasms, which are often triggered by stimuli such as noise, touch, and emotional distress. These spasms can be painful and debilitating, significantly impacting the quality of life of those affected.
Etiology and Pathophysiology
The exact cause of Stiff Person Syndrome is not well understood, but it is believed to be an autoimmune disorder. Research suggests that the immune system mistakenly attacks parts of its own nervous system, specifically affecting glutamic acid decarboxylase (GAD), an enzyme crucial for the production of the neurotransmitter gamma-aminobutyric acid (GABA). GABA is important for regulating muscle tone, and its deficiency can lead to the symptoms observed in SPS.
Symptoms
The primary symptoms of Stiff Person Syndrome include:
Muscle stiffness and rigidity: This usually begins in the trunk and can spread to the limbs.
Painful spasms: These can be spontaneous or triggered by external stimuli such as sounds, touch, or emotional distress.
Deformities and abnormal postures: Over time, the continuous muscle rigidity can lead to fixed deformities and abnormal posture.
Heightened sensitivity (hyperexplexia): Sudden noises or unexpected touches can provoke exaggerated startle responses.
Functional impairment: Mobility may be significantly affected, and individuals with severe cases might become wheelchair-bound.
Diagnosis
Diagnosing SPS can be challenging due to its rarity and the variability of its presentation. The diagnosis typically involves:
Clinical evaluation: Assessing the symptoms and medical history.
Blood tests: Looking for antibodies such as anti-GAD (glutamic acid decarboxylase) antibodies.
Electromyography (EMG): This test measures electrical activity in muscles and can detect abnormalities characteristic of SPS.
MRI and other imaging techniques: These are used to rule out other neurological disorders.
Treatment
There is currently no cure for Stiff Person Syndrome, but treatments are available that can help manage symptoms:
Muscle relaxants and anti-spasmodic medications: Such as baclofen, diazepam, or botulinum toxin.
Immunotherapy: Treatments such as IVIG (intravenous immunoglobulin), plasmapheresis, or immunosuppressants can be effective in managing autoimmune aspects of the disease.
Physical therapy: Helps maintain mobility and manage stiffness.
Pain management: Techniques and medications to manage chronic pain.
Prognosis
The prognosis of Stiff Person Syndrome varies. Some individuals may experience a gradual worsening of symptoms, while others might maintain a stable condition. Early diagnosis and treatment can improve the quality of life and potentially slow the progression of the disease.
Challenges and Ongoing Research
Given its rarity, SPS is often under-recognized, leading to delays in diagnosis and treatment. Ongoing research is focused on better understanding the pathophysiology of the disease, developing more effective treatments, and improving diagnostic tools.
What I’ve read this week
*Click on the pic to read*