Hi everyone,
Welcome to all our new subscribers who joined us this week! We're thrilled to have you as part of The Weekly Pill community. Your readership means the world to us. If you could kindly share this newsletter with your friends or coworkers, we would greatly appreciate your support! This resource holds value for a wide range of individuals, including venture capitalists, students, professionals at all levels within pharmaceutical companies, and anyone with a curious mindset eager to learn. Your assistance in this endeavor is greatly appreciated.
I sincerely hope you enjoy reading our newsletter and that it adds value to your understanding of the biotech world. Wishing each of you a blessed week ahead, and I look forward to connecting with you all again next week for another update.
If you really enjoy what we're doing here, consider becoming a paid subscriber to encourage us and gain access to the paid section, which includes insights on upcoming catalysts next week, the most shorted biotech stocks, earnings, market comments, notes and M&A comments. Now let’s dive into last week recap!
Table of contents
How the market performed this week
Licenses / Partnerships
Clinical trials
Financing
RIF
Disease of the week
What I’ve read this week
Paid Content Section
Upcoming Catalysts Next Week
Reminders
Biotech Valuation
Most Shorted Biotech Stock as of This Week
Market
Notes from analysts
Venture Capital Market
M&A
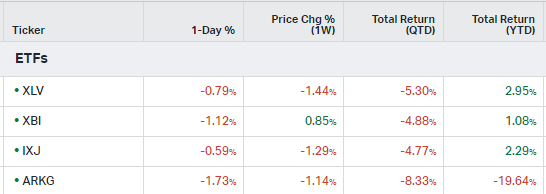
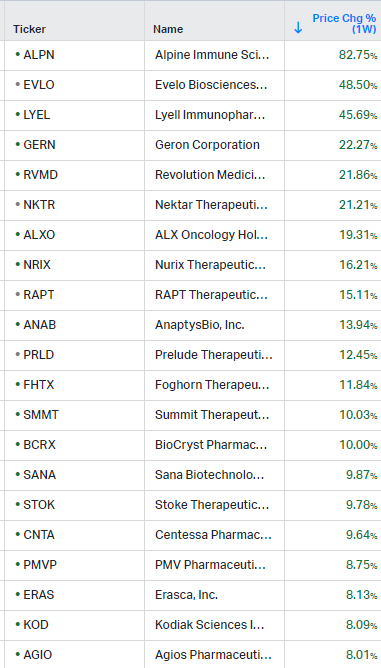
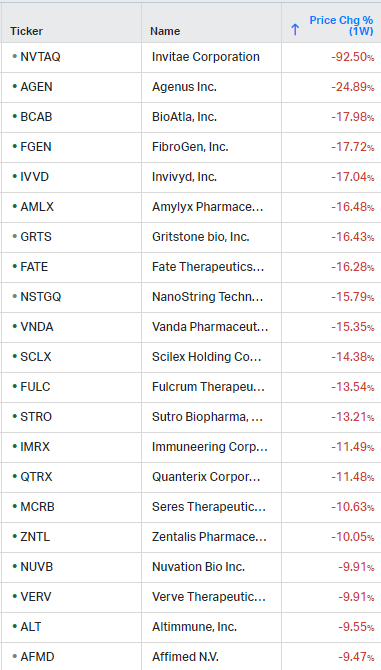
How the market performed this week
ETFs
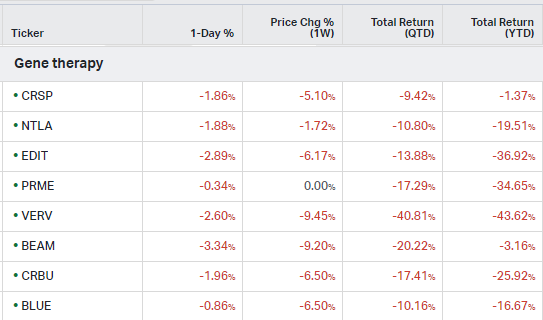
Gene Therapy
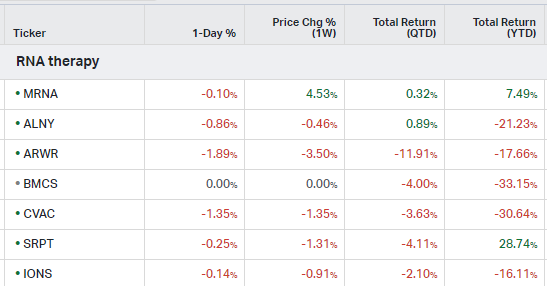
RNA Therapy
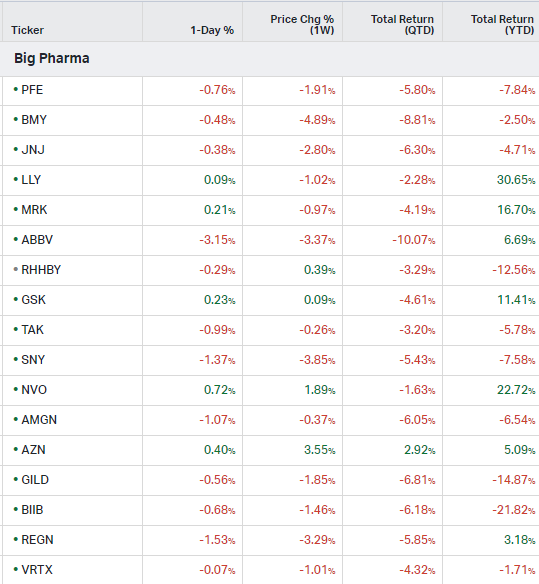
Big Pharma
Gainers / Decliners this week in biotech
News of the week
Licenses/Partnerships
Harmony Biosciences Holdings, Inc.
Lead Company: HRMY
Event Type: Partnership - Licensing Deal
Event Phase: Preclinical
Drug: TPM-1116
Other Names: TPM 1116, TPM1116
Disease Group: Neurology
Target: Hypocretin/orexin receptor
LOA: Not specified
Source Link: Link
Novartis AG
Lead Company: NVS
Event Type: Partnership - Licensing Deal
Event Phase: II
Drug: ARV-766
Other Names: ARV766, ARV 766
Disease Group: Oncology
Target: Androgen receptors
LOA: 11%
Source Link: Link
AbbVie Inc.
Lead Company: ABBV
Event Type: Partnership - Acquisition Announcement
Event Phase: II
Drug: ALPN-101
Other Names: vlgD Localized ICOS/CD28 Agonist (Alpine), ALPN101, ALPN 101
Disease Group: Autoimmune/immunology
Target: Cluster of Differentiation 28 (CD28) /ICOS and B7RP-1 Pathway, Immune System
LOA: 19%
Source Link: Link
Alpine Immune Sciences Inc.
Lead Company: ALPN
Event Type: Partnership - Acquisition Announcement
Event Phase: II
Drug: ALPN-303
Other Names: ALPN 303, ALPN303, ALPN-303, dual BAFF/APRIL inhibitors
Disease Group: Renal
Target: APRIL, B-cell activating factor (BAFF)/B-lymphocyte stimulator (BLyS)
LOA: 15%
Source Link: Link
Nuvation Bio, Inc.
Lead Company: NUVB
Event Type: Partnership - Acquisition Closed
Event Phase: II
Drug: AB-106
Other Names: DS6051, DS 6051, DS-6051, DS6051b, AB 106, AB106, Taletrectinib, IBI344, IBI-344, IBI 344
Disease Group: Oncology
Target: ROS kinase, Trk (Tropomyosin Receptor Kinase) Receptors
LOA: 11%
Source Link: Link
Nurix Therapeutics, Inc.
Lead Company: NRIX
Event Type: Partnership - Amendment/Restructuring
Event Phase: Preclinical
Drug: E3 Ligase Protein Degradation Program (Nurix/Sanofi)
Other Names: DELigase Platform
Disease Group: Not Specified
Target: E3 ubiquitin ligase
LOA: Not specified
Source Link: Link
Transcenta Holding Ltd.
Lead Company: 6628
Event Type: Partnership - Announcement
Event Phase: I
Drug: TST-001
Other Names: TST001, TST 001, Humanized, Low-fucose Anti-Claudin 18.2 IgG1 Antibody
Disease Group: Oncology
Target: Claudin 18 (CLDN18)
LOA: 5%
Source Link: Link
Clinical trials (LOA=likelihood of approval)
4SC AG
Lead Company: VSC
Event Phase: II
Trial Name: Phase II - RESMAIN
Drug: Resminostat
Disease Group: Oncology
Indication: Cutaneous T-Cell Lymphoma (CTCL) - NHL
Target: Histone Deacetylase (HDAC)
LOA: 15%
Source Link: Link
MEI Pharma, Inc.
Lead Company: MEIP
Event Phase: Suspended
Trial Name: Phase Ib - w/Avastin
Drug: ME-344
Disease Group: Oncology
Indication: Colorectal Cancer (CRC)
Target: Mammalian Target of Rapamycin (mTOR)/mTORC, Mitochondria
LOA: Not specified
Source Link: Link
Enliven Therapeutics, Inc.
Lead Company: ELVN
Event Phase: I
Trial Name: Phase Ia/Ib - ELVN-001-101
Drug: ELVN-001
Disease Group: Oncology
Indication: Chronic Myelogenous Leukemia (CML)
Target: BCR-ABL Fusion Protein
LOA: 5%
Source Link: Link
CorMedix Inc.
Lead Company: CRMD
Event Phase: Approved
Trial Name: Preclinical Studies
Drug: DefenCath
Disease Group: Cardiovascular
Indication: Catheter Complications
Target: Calcium, Cell wall, Coagulation Factor X, Thrombin (Coagulation Factor IIa)
LOA: 100%
Source Link: Link
Lipocine Inc.
Lead Company: LPCN
Event Phase: II
Trial Name: Phase II - Prospective Study
Drug: LPCN 2401
Disease Group: Metabolic
Indication: Obesity
Target: Androgen receptors
LOA: 25%
Source Link: Link
Aprea Therapeutics, Inc.
Lead Company: APRE
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: ATRN-333
Disease Group: Oncology
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Target: Unknown
LOA: Not specified
Source Link: Link
Aprea Therapeutics, Inc.
Lead Company: APRE
Event Phase: IND
Trial Name: Preclinical Studies
Drug: APR-1051
Disease Group: Oncology
Indication: Ovarian Cancer
Target: Wee1 Inhibitor
LOA: Not specified
Source Link: Link
BriaCell Therapeutics Corp.
Lead Company: BCT
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: Bria-Pros
Disease Group: Oncology
Indication: Prostate Cancer
Target: Granulocyte-Macrophage CSF (GM-CSF), Immune System, T lymphocytes
LOA: Not specified
Source Link: Link
Provectus Biopharmaceuticals, Inc.
Lead Company: PVCT
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: PV-10
Disease Group: Oncology
Indication: Head and Neck Cancer
Target: Immune System
LOA: Not specified
Source Link: Link
Immunome Inc.
Lead Company: IMNM
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: IM-3050
Disease Group: Oncology
Indication: Solid Tumors
Target: Fibroblast Activation Protein (FAP)
LOA: Not specified
Source Link: Link
Innate Pharma S.A.
Lead Company: IPHA
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: IPH45
Disease Group: Oncology
Indication: Cancer
Target: Nectin-4, Topoisomerase I (Topo-I)
LOA: Not specified
Source Link: Link
MAIA Biotechnology, Inc.
Lead Company: MAIA
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: THIO
Disease Group: Oncology
Indication: Brain Cancer (Malignant Glioma; AA and glioblastoma (GBM))
Target: DNA synthesis, Telomeres
LOA: Not specified
Source Link: Link
Medicenna Therapeutics Corp.
Lead Company: MDNA
Event Phase: II
Trial Name: Phase I/II - ABILITY-1
Drug: MDNA11
Disease Group: Oncology
Indication: Solid Tumors
Target: IL-2 Receptor (IL-2R)
LOA: 11%
Source Link: Link
Apollomics, Inc.
Lead Company: APLM
Event Phase: II
Trial Name: Preclinical Studies
Drug: APL-101
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Hepatocyte growth factor receptor (c-Met, HGFR)
LOA: 11%
Source Link: Link
Mural Oncology plc
Lead Company: MURA
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: IL-12 Program (Mural Oncology)
Disease Group: Oncology
Indication: Cancer
Target: IL-12 (Interleukin-12) and IL-12 receptor
LOA: Not specified
Source Link: Link
IO Biotech Inc.
Lead Company: IOBT
Event Phase: II
Trial Name: Preclinical Studies
Drug: IO102-IO103
Disease Group: Oncology
Indication: Solid Tumors
Target: IDO (Indoleamine 2,3-dioxygenase), Programmed death-ligand 1 (PD-L1)
LOA: 11%
Source Link: Link
Molecular Templates, Inc.
Lead Company: MTEM
Event Phase: I
Trial Name: Phase I - Dose Escalation (U.S.)
Drug: MT-6402
Disease Group: Oncology
Indication: Solid Tumors
Target: Aldehyde Dehydrogenase 2 (ALDH2), Programmed death-ligand 1 (PD-L1), T lymphocytes
LOA: 5%
Source Link: Link
NeoImmuneTech, Inc.
Lead Company: 950220
Event Phase: II
Trial Name: Preclinical Studies
Drug: HyLeukin
Disease Group: Oncology
Indication: Colorectal Cancer (CRC)
Target: IL-7 (Interleukin-7) and IL-7 receptor (IL-7R)
LOA: 11%
Source Link: Link
Elevation Oncology, Inc.
Lead Company: ELEV
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: HER3-ADC Program (Elevation Oncology)
Disease Group: Oncology
Indication: Cancer
Target: ErbB3/HER3
LOA: Not specified
Source Link: Link
BioNTech SE
Lead Company: BNTX
Event Phase: II
Trial Name: Phase I - LuCa-MERIT-1
Drug: BNT116
Disease Group: Oncology
Indication: Non-Small Cell Lung Cancer (NSCLC)
Target: Immune System
LOA: 11%
Source Link: Link
ALX Oncology, Inc.
Lead Company: ALXO
Event Phase: Suspended
Trial Name: Phase Ib/II - ASPEN-02 (w/Azacitidine)
Drug: Evorpacept
Disease Group: Oncology
Indication: Myelodysplastic Syndrome (MDS)
Target: Cluster of Differentiation 47 (CD47)
LOA: Not specified
Source Link: Link
Boundless Bio, Inc.
Lead Company: BOLD
Event Phase: II
Trial Name: Phase I/II - POTENTIATE
Drug: BBI-355
Disease Group: Oncology
Indication: Solid Tumors
Target: Cell Cycle Checkpoint Kinase 1 (Chk1)
LOA: 11%
Source Link: Link
Silence Therapeutics plc
Lead Company: SLN
Event Phase: I
Trial Name: Phase I - APOLLO
Drug: Zerlasiran
Disease Group: Cardiovascular
Indication: Dyslipidemia / Hypercholesterolemia
Target: Lipoprotein (a) [Lp(a)]
LOA: 6%
Source Link: Link
Theratechnologies Inc.
Lead Company: THTX
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: Sudocetaxel Zendusortide
Disease Group: Oncology
Indication: Melanoma
Target: Microtubules (Tubulin), Sortilin 1 (SORT1)
LOA: Not specified
Source Link: Link
Theratechnologies Inc.
Lead Company: THTX
Event Phase: Preclinical
Trial Name: Preclinical Studies
Drug: Sudocetaxel Zendusortide
Disease Group: Oncology
Indication: Triple-Negative Breast Cancer (TNBC)
Target: Microtubules (Tubulin), Sortilin 1 (SORT1)
LOA: Not specified
Source Link: Link
Photocure ASA
Lead Company: PHO
Event Phase: Approved
Trial Name: Phase III - Bridging Study (China)
Drug: Cevira
Disease Group: Oncology
Indication: Bladder Cancer - Imaging
Target: Unknown
LOA: 100%
Source Link: Link
Financing events
22nd Century Group (NAS: XXII)
Description: Biotechnology company focusing on alkaloid plant technologies to improve health and wellness.
Verticals: AgTech, Cannabis, HealthTech, Life Sciences, Manufacturing, TMT
Deal Date: 09-avr-2024
Deal Type: Public Investment 2nd Offering
Deal Synopsis: Raised $3.97 million in its second public offering on the Nasdaq stock exchange.
Investors: N/A
Deal Size: $3.97 million
Acrivon Therapeutics (NAS: ACRV)
Description: Clinical-stage biopharmaceutical company developing oncology medicines.
Verticals: Artificial Intelligence & Machine Learning, Life Sciences, Oncology
Deal Date: 09-avr-2024
Deal Type: PIPE
Deal Synopsis: In talks to receive $130 million of development capital through a private placement.
Investors: Acorn Bioventures, Paradigm BioCapital, Perceptive Advisors, RA Capital Management, Sands Capital, Surveyor Capital
Deal Size: $130.00 million
Alpine Immune Sciences (NAS: ALPN)
Description: Clinical-stage biopharmaceutical company focused on developing protein-based immunotherapies.
Verticals: Life Sciences, Oncology
Deal Date: 10-avr-2024
Deal Type: Merger/Acquisition
Deal Synopsis: Acquired by Vertex Pharmaceuticals for approximately $4.9 billion.
Investors: Vertex Pharmaceuticals
Deal Size: $4.9 billion
Bionano (NAS: BNGO)
Description: Life sciences instrumentation company in the genome analysis space.
Verticals: Life Sciences, Oncology
Deal Date: 08-avr-2024
Deal Type: Public Investment 2nd Offering
Deal Synopsis: Raised $7.48 million in its second public offering on the Nasdaq stock exchange.
Investors: N/A
Deal Size: $7.48 million
Century Therapeutics (NAS: IPSC)
Description: Biotechnology company leveraging adult stem cells to develop advanced cell therapy products.
Verticals: Life Sciences, Oncology
Deal Date: 11-avr-2024
Deal Type: PIPE
Deal Synopsis: In talks to receive approximately $60 million of development capital through a private placement.
Investors: Adage Capital Management, Bain Capital Life Sciences, Boxer Capital, Casdin Capital, DAFNA Capital Management, Octagon Capital Advisors, Superstring Capital Management, Venrock
Deal Size: $60.00 million
Reduction in force (RIF)
April 10 - Genentech: Genentech is trimming roughly 3% of its workforce across "several departments," a spokesperson confirmed, after the layoffs were first reported by Endpoints News. The larger biotech's parent company Roche announced cuts to its product development team earlier this year.
April 9 - Novartis: The Big Pharma has revealed plans to shake up its global development group over the next few years, with intentions to cut around 440 development positions in Switzerland, plus up to 240 roles in the U.S., a spokesperson said. Story
April 5 - Sanofi: The pharma is setting out plans for a new "simplified R&D structure" that zooms in on immunology and means an undisclosed number of staffers will be losing their jobs. A Sanofi spokesperson declined to disclose to Fierce about how many employees will be impacted in total, restating that prioritizing immunology, “means that we are reallocating and refocusing resources to accelerate our investments in programs with high potential.” Story
April 5 - Boehringer Ingelheim: Since the pharma's Humira biosimilar—dubbed Cyltezo—has struggled to gain traction, Boehringer is pruning its ranks. The company has revealed plans to lay off an unknown number of staffers from its customer-facing teams for Cyltezo as part of a pivot toward a hybrid in-person and virtual sales model by June 30. Story
April 4 - Amylyx Pharmaceuticals: After Relyvrio failed a confirmatory trial, Amylyx has pulled the ALS therapy off the market and is laying off about 70% of staff. As of the end of 2023, Amylyx had 384 full-time employees, according to an annual securities filing. The cuts will leave the company with about 100 remaining workers. Story
Disease of the week
Eosinophilic Esophagitis (EoE) is a chronic immune-mediated disorder characterized by inflammation of the esophagus. The esophagus is the muscular tube that carries food from the mouth to the stomach. In EoE, the inflammation is driven by an allergic reaction to certain foods or environmental triggers.
Here's a breakdown of key aspects of Eosinophilic Esophagitis:
Symptoms:
Difficulty Swallowing (Dysphagia): This is the most common symptom and can range from mild to severe.
Food Impaction: Solid food may become stuck in the esophagus, causing chest pain or discomfort.
Reflux Symptoms: These include heartburn, regurgitation, and chest pain.
Nausea and Vomiting: Some individuals may experience these symptoms, particularly after eating.
Failure to Thrive (in children): EoE can lead to poor growth and development in children.
Diagnosis:
Endoscopy: A procedure in which a flexible tube with a camera (endoscope) is inserted into the esophagus to visualize any inflammation or abnormalities.
Biopsy: During endoscopy, small tissue samples (biopsies) are taken from the lining of the esophagus. These are examined under a microscope to look for high levels of eosinophils, a type of white blood cell indicative of allergic inflammation.
Elimination Diet: This involves removing specific foods from the diet to determine if they are triggers for EoE. Foods commonly eliminated include dairy, wheat, eggs, soy, nuts, and seafood.
Allergy Testing: Skin prick tests or blood tests may be performed to identify specific food allergies or environmental allergens.
Treatment:
Dietary Management: Elimination diets may be prescribed based on the results of allergy testing or clinical observation. This involves avoiding trigger foods identified through testing or clinical experience.
Medications:
Proton Pump Inhibitors (PPIs): These medications reduce stomach acid production and may help alleviate symptoms.
Topical Steroids: Swallowed steroid medications or steroid inhalers used off-label may be prescribed to reduce inflammation in the esophagus.
Dilation: In cases of severe narrowing (strictures) of the esophagus due to scarring from inflammation, a procedure called dilation may be performed to widen the esophagus.
Monitoring: Regular endoscopies and biopsies may be recommended to monitor the effectiveness of treatment and assess for any complications.
Prognosis:
Eosinophilic Esophagitis is a chronic condition that requires long-term management. With appropriate treatment, most individuals with EoE can achieve control of their symptoms and prevent complications such as strictures. However, it's essential to work closely with healthcare providers to develop a personalized treatment plan and monitor for any changes in symptoms or disease progression.
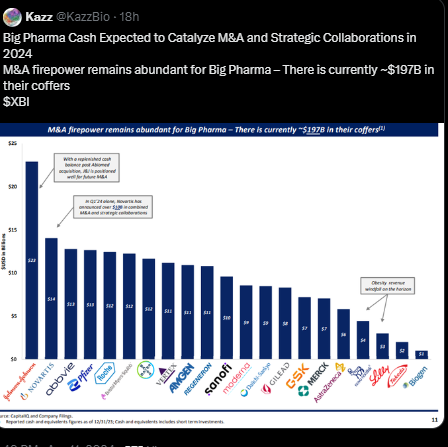
What I’ve read this week
*Click on the pic to read*